Abstract
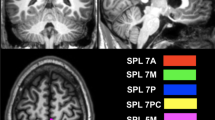
The present paper concentrates on the impact of visual attention task on structure of the brain functional and effective connectivity networks using coherence and Granger causality methods. Since most studies used correlation method and resting-state functional connectivity, the task-based approach was selected for this experiment to boost our knowledge of spatial and feature-based attention. In the present study, the whole brain was divided into 82 sub-regions based on Brodmann areas. The coherence and Granger causality were applied to construct functional and effective connectivity matrices. These matrices were converted into graphs using a threshold, and the graph theory measures were calculated from it including degree and characteristic path length. Visual attention was found to reveal more information during the spatial-based task. The degree was higher while performing a spatial-based task, whereas characteristic path length was lower in the spatial-based task in both functional and effective connectivity. Primary and secondary visual cortex (17 and 18 Brodmann areas) were highly connected to parietal and prefrontal cortex while doing visual attention task. Whole brain connectivity was also calculated in both functional and effective connectivity. Our results reveal that Brodmann areas of 17, 18, 19, 46, 3 and 4 had a significant role proving that somatosensory, parietal and prefrontal regions along with visual cortex were highly connected to other parts of the cortex during the visual attention task. Characteristic path length results indicated an increase in functional connectivity and more functional integration in spatial-based attention compared with feature-based attention. The results of this work can provide useful information about the mechanism of visual attention at the network level.














Similar content being viewed by others
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Bar M (2003) A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci 15(4):600–609
Battelli L, Cavanagh P, Martini P, Barton JJS (2003) Bilateral deficits of transient visual attention in right parietal patients. Brain 126(10):2164–2174
Battelli L, Pascual-Leone A, Cavanagh P (2007) The “when” pathway of the right parietal lobe. Trends Cogn Sci 11(5):204–210
Behroozi M, Daliri MR (2012) Software tools for the analysis of functional magnetic resonance imaging. Basic Clin Neurosci 3:71–83
Behroozi M, Daliri MR (2014a) RDLPFC area of the brain encodes sentence polarity: a study using fMRI. Brain Imaging Behav 9:178–189
Behroozi M, Daliri MR (2014b) Predicting brain states associated with object categories from fMRI data. J Integr Neurosci 13:645–667
Behroozi M, Daliri MR, Boyaci H (2011) Statistical analysis methods for the fMRI data. Basic Clin Neurosci 2:67–74
Berberovic N, Pisella L, Morris AP, Mattingley JB (2004) Prismatic adaptation reduces biased temporal order judgements in spatial neglect. NeuroReport 15(7):1199–1204
Bhattacharya J, Petsche H (2005) Phase synchrony analysis of EEG during music perception reveals changes in functional connectivity due to musical expertise. Signal Process 85:2161–2177
Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang D-U (2005) structure and dynamics. Phys Rep 424:175–308
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108(3):624–652
Brefczynski JA, DeYoe EA (1999) A physiological correlate of the ‘spotlight’ of visual attention. Nat Neurosci 2(4):370–374
Brillinger DR (2001) Time series: data analysis and theory. Society for Industrial and Applied Mathematics, Philadephia
Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler S (2004) Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci 101(26):9849–9854
Buckner LR (2010) Human functional connectivity: new tools, unresolved questions. Proc Natl Acad Sci 107(24):10769–10770
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4(6):215–222
Carrasco M (2011) Visual attention: the past 25 years. Vis Res 51(13):1484–1525
Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA (1998) A common network of functional areas for attention and eye movements. Neuron 21(4):761–773
Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000) Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3(3):292–297
Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A (2005) Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci 8(11):1603–1610
Corchs S, Deco G (2004) Feature-based attention in human visual cortex: simulation of fMRI data. NeuroImage 21:36–45
Cornelis JS, Jaap CR (2007) Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys 1:3
Coull JT, Nobre AC (1998) Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 18(18):7426–7435
Daliri MR, Kozyrev V, Treue S (2016) Attention enhances stimulus representations in macaque visual cortex without affecting their signal-to-noise level. Sci Rep 6:27666
Davis B, Christie J, Rorden C (2009) Temporal order judgments activate temporal parietal junction. J Neurosci 29(10):3182–3188
Decaix C, Chokron S, Bartolomeo P, Sieroff E (2001) Modulating the attentional bias in unilateral neglect: the effects of the strategic set. Exp Brain Res 137(3-4):432–444
Desimone R, Duncan J (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18:193–222
Dimitriadis SI, Salis C, Tarnanas I, Linden DE (2017) Topological filtering of dynamic functional brain networks unfolds informative chronnectomics: a novel data-driven thresholding scheme based on orthogonal minimal spanning trees (OMSTs). Front Neuroinform 11:28
Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA et al (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci 104(26):11073–11078
Duncan J (1980) The locus of interference in the perception of simultaneous stimuli. Psychol Rev 87:272–300
Duncan J, Humphreys GW (1989) Visual search and stimulus similarity. Psychol Rev 96:433–458
Fagiolo G (2007) Clustering in complex directed networks. Phys Rev E Stat Nonlin Soft Matter Phys 76:026107
Fenske MJ, Aminoff E, Gronau N, Bar M (2006) Top-down facilitation of visual object recognition: object-based and context-based contributions. Prog Brain Res 155:3–21
Gandhi SP, Heeger DJ, Boynton GM (1999) Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci 96(6):3314–3319
Geweke J (1982) Measurement of linear dependence and feedback between multiple time series. J Am Stat Assoc 77(378):304–313
Gharabaghi A, Fruhmann M, Tatagiba M, Karnath HO (2006) The role of the right superior temporal gyrus in visual search-insights from intraoperative electrical stimulation. Neuropsychologia 44(12):2578–2581
Gramann K, Töllner T, Müller HJ (2010) Dimension-based attention modulates early visual processing. Psychophysiology 47(5):968–978
Granger C (1969) Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37:424–438
Hahn B, Ross TJ, Stein EA (2006) Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage 32(2):842–853
Jehee JFM, Brady DK, Tong F (2011) Attention improves encoding of task-relevant features in the human visual cortex. J Neurosci 31:8210–8219
Kamitani Y, Tong F (2005) Decoding the visual and subjective contents of the human brain. Nat Neurosci 8:679–685
Kamitani Y, Tong F (2006) Decoding seen and attended motion directions from activity in the human visual cortex. NCBI 16(11):1096–1102
Kastner S, Ungerleider LG (2000) Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23:315–341
Kastner S, Pinsk MA, De WP, Desimone R, Ungerleider LG (1999) Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22(4):751–761
Kimchi R, Behrmann M, Olson CR (2003) Perceptual organization in vision: behavioral and neural perspectives. Psychology Press, Hove
Latora V, Marchiori M (2003) Economic small-world behavior in weighted networks. Eur Phys J B 32:249–263
Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA (2003) Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 15(7):1028–1038
Liao W, Ding J, Marinazzo D, Xu Q, Wang Z, Yuan C, Zhang Z, Lu G, Chen H (2011) Smallworld directed networks in the human brain: multivariate Granger causality analysis of resting-state fMRI. NeuroImage 54:2683–2694
Ling S, Pratte MS, Tong F (2015) Attention alters orientation processing in the human lateral geniculate nucleus. Nat Neurosci 18:496–498
Lu Z, Sperling G (2001) Three-systems theory of human visual motion perception: review and update. J Opt Soc Am 18(9):2331–2370
Luck SJ, Hillyard SA, Mouloua M, Hawkins HL (1996) Mechanisms of visual-spatial attention: resource allocation or uncertainty reduction. J Exp Psychol Hum Percept Perform 22(3):725–737
Marchini JL, Ripley BD (2000) A new statistical approach to detecting significant activation in functional MRI. NeuroImage 12:366–380
Mechelli A, Price CJ, Friston KJ, Ishai A (2004) Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cereb Cortex 14(11):1256–1265
Müller HJ, Heller D, Ziegler J (1995) Visual search for singleton feature targets within and across feature dimensions. Percept Psychophys 57(1):1–17
Newman MEJ (2003) The structure and function of complex networks. SIAM Rev 45:167–256
Peelen MV, Kastner S (2011) A neural basis for real-world visual search in human occipitotemporal cortex. Proc Natl Acad Sci 108(29):12125–12130
Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME (2007) Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci 27(44):11912–11924
Poppel E (1997) A hierarchical model of temporal perception. Trends Cogn Sci 1(2):56–61
Posner MI, Petersen SE (1990) The attention system of the human brain. NCBI 13:25–42
Robertson IH, Mattingley JB, Rorden C, Driver J (1998) Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature 395(6698):169–172
Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ et al (1999) Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci 11(2):135–152
Schwartz G (1978) Estimating the dimension of a model. Ann Stat 5(2):461–464
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356
Seif Z, Daliri MR (2015) Evaluation of local field potential signals in decoding of visual attention. Cogn Neurodyn 9(5):509–522
Seth AK (2010) A MATLAB toolbox for Granger causal connectivity analysis. J Neurosci Methods 186:262–273
Shulman GL, Pope DLW, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M (2010) Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30(10):3640–3651
Sporns O, Honey CJ (2006) Small worlds inside big brains. Proc Natl Acad Sci 103:19219–19220
Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J (2006) Predictive codes for forthcoming perception in the frontal cortex. Science 314(5803):1311–1314
Verstraten FA, Cavanagh P, Labianca AT (2000) Limits of attentive tracking reveal temporal properties of attention. Vis Res 40(26):3651–3664
Watts DJ, Strogatz SH (1998) Collective dynamics of “small-world” networks. Nature 393:440–442
Wiener N (1956) The theory of prediction. In: Beckenbach EF (ed) Modern mathematics for engineers. McGraw-Hill, New York
Wolfe JM, Horowitz TS (2004) What attributes guide the deployment of visual attention and how do they do it? Nat Rev Neurosci 5(6):495–501
Acknowledgements
We would like to thank the team (especially Prof. Boyaci) from UMRAM laboratory, Bilkent University, Ankara, Turkey, for providing the infrastructure for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Parhizi, B., Daliri, M.R. & Behroozi, M. Decoding the different states of visual attention using functional and effective connectivity features in fMRI data. Cogn Neurodyn 12, 157–170 (2018). https://doi.org/10.1007/s11571-017-9461-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-017-9461-1