Abstract
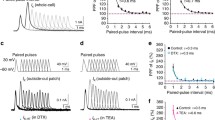
The replenishment rates estimated from the recovery of synaptic efficacy following synaptic depression are known to be widely scattered. Given the importance of the replenishment during stimulation, especially if it is prolonged, it is important to better understand what influences the recovery of the synaptic efficacy following stimulation. We fit a two-pool model of vesicular secretion to the changes of the excitatory post-synaptic currents recorded in CA1 neurons of rat hippocampal slices to determine how the model parameters change during, and following, long stimulation. The replenishment rate at the end of stimulation inducing synaptic depression differs greatly from that at the beginning of stimulation. It decreases progressively and rapidly (by ~75 % and with a time constant of <10 s) during stimulation, and this is followed by a similarly fast recovery (time constant of ~10 s), but to a steady-state that is approximately twice as large as its pre-stimulation value. Both [Ca++]o and the duration of long stimulation influence the recovery of the replenishment rate. Its new steady-state is significantly higher, if either [Ca++]o is higher or stimulation longer, but the recovery of the replenishment rate becomes clearly slower if [Ca++]o is higher, and faster if stimulation is longer. Many factors thus influence the recovery of the replenishment rate and of the synaptic efficacy, but the stimulation induced [Ca++]i accumulation cannot explain the change of the replenishment rate during recovery. Finally, okadaic acid, which speeds up vesicular trafficking, does not alter the recovery of the replenishment rate. The vesicular replenishment of the RRP following stimulation is thus not likely to be associated with significant vesicular movement.









Similar content being viewed by others
References
Aristizabal F, Glavinovic MI (2004) Simulation and parameter estimation of dynamics of synaptic depression. Biol Cybern 90:3–18
Atmanspacher H, Rotter S (2008) Interpreting neurodynamics: concepts and facts. Cogn Neurodyn 2:297–318
Betz WJ, Henkel AW (1994) Okadaic acid disrupts clusters of synaptic vesicles in frog motor nerve terminals. J Cell Biol 124:843–854
Birks R, MacIntosh FC (1961) Acetylcholine metabolism of a sympathetic ganglion. Can J Biochem Physiol 39:787–827
Blanton MG, Lom Turcom JJ, Kriegstein AR (1989) Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Meth 30:203–210
Bui L, Glavinovic MI (2013) Synaptic activity slows vesicular replenishment at excitatory synapses of rat hippocampus. Cogn Neurodyn. doi:10.1007/s11571-012-9232-y
Chirilian PM (1969) Basic network theory. McGraw-Hill, New York
Christensen BN, Martin AR (1970) Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol 210:933–945
Clopath C (2012) Synaptic consolidation: an approach to long-term learning. Cogn Neurodyn 6:251–257
Coleman TF, Li Y (1996a) A reflective Newton method for minimizing a quadratic function subject to bounds on some of the variables. SIAM J Optim 6:1040–1058
Coleman TF, Li Y (1996b) An interior, trust region approach for nonlinear minimization subject to bounds. SIAM J Optim 6:418–445
Del Castillo J, Katz B (1954) Statistical factors involved in neuromuscular facilitation and depression. J Physiol 124:574–585
Dittman JS, Regehr WG (1998) Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci 18:6147–6162
Gabriel T, García-Perez E, Mahfooz K, Goni J, Martínez-Turrillas R, Perez-Otano I, Lo DC, Wesseling JF (2011) A new kinetic framework for synaptic vesicle trafficking tested in synapsin knock-outs. J Neurosci 31:11563–11577
Gaffield MA, Rizzoli SO, Betz WJ (2006) Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron 51:317–325
Heinemann C, von RL, Chow RH, Neher E (1993) A two-step model of secretion control in neuroendocrine cells. Pflugers Arch 424:105–112
Kavalali ET (2007) Multiple vesicle recycling pathways in central synapses and their impact on neurotransmission. J Physiol 585: 669–679
Krnjevic K, Bui L, Glavinovic MI (2008) Vesicular dynamics is time dependent at excitatory synapses of rat hippocampus. Soc Neurosci Abstr 33:432.20/E26
Kruckenberg P, Sandweg R (1968) An analog model for acetylcholine release by motor nerve endings. J Theor Biol 19:327–332
Kuroda S, Fukushima Y, Yamaguti Y, Tsukada M, Tsuda I (2009) Iterated function systems in the hippocampal CA1. Cogn Neurodyn 3:205–222
Malchiodi-Albedi F, Petrucci TC, Picconi B, Iosi F, Falchi M (1997) Protein phosphatase inhibitors induce modification of synapse structure and tau hyperphosphorylation in cultured rat hippocampal neurons. J Neurosci Res 48:425–438
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
Morales M, Colicos MA, Goda Y (2000) Actin-dependent regulation of neurotransmitter release at central synapses. Neuron 27:539–550
Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ (1993) The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron 11:713–724
Satel J, Trappenberg T, Fine A (2009) Are binary synapses superior to graded weight representations in stochastic attractor networks? Cogn Neurodyn 3:243–250
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21
Shakiryanova D, Klosem MK, Zhoum Y, Gum T, Deitcherm DL, Atwood HL, Hewes RS, Levitan ES (2007) Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci 27:7799–7806
Shtrahman M, Yeung C, Nauen DW, Bi GQ, Wu XL (2005) Probing vesicle dynamics in single hippocampal synapses. Biophys J 89:3615–3627
Stevens CF, Tsujimoto T (1995) Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci USA 92:846–849
Stevens CF, Wesseling JF (1998) Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron 21:415–424
Stevens CF, Wesseling JF (1999) Identification of a novel process limiting the rate of synaptic vesicle cycling at hippocampal synapses. Neuron 24:1017–1028
Taniike N, Lu YF, Tomizawa K, Matsui H (2008) Critical differences in magnitude and duration of N-methyl-d-aspartate (NMDA) receptor activation between long-term potentiation (LTP) and long-term depression (LTD) induction. Acta Med Okayama 62:21–28
Wang LY, Kaczmarek LK (1998) High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394:384–388
Wang Y, Manis PB (2008) Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol 100:1255–1264
Weis S, Schneggenburger R, Neher E (1999) Properties of a model of Ca(++)-dependent vesicle pool dynamics and short term synaptic depression. Biophys J 77:2418–2429
Wesseling JF, Lo DC (2002) Limit on the role of activity in controlling the release-ready supply of synaptic vesicles. J Neurosci 22:9708–9720
Zola-Morgan S, Squire LR, Amaral DG (1986) Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 6:2950–2967
Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405
Acknowledgments
This work was supported by the grant from the Natural Sciences and Engineering Research Council of Canada and Canadian Heart and Stroke Foundation to M.I.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bui, L., Glavinović, M.I. Recovery of vesicular storage and release parameters after high frequency stimulation in rat hippocampus. Cogn Neurodyn 7, 311–323 (2013). https://doi.org/10.1007/s11571-012-9240-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-012-9240-y