Abstract
Purpose
Realistic soft tissue deformation modeling and haptic rendering for surgical simulation require accurate knowledge of tissue material characteristics. Biomechanical experiments on porcine tissue were performed, and a reduced quasi-linear viscoelastic model was developed to describe the strain-dependent relaxation behavior of the arterial wall. This information is used in surgical simulation to provide a realistic sensation of reduction in strength when the user holds a virtual blood vessel strained at different levels.
Materials and methods
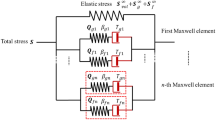
Twelve pieces of porcine abdominal artery were tested with uniaxial elongation and relaxation test in both circumferential and longitudinal directions. The mechanical property testing system consists of automated environment control, testing, and data collection mechanism. A combined logarithm and polynomial strain energy equation was applied to model the elastic response of the specimens. The reduced relaxation function was modified by integrating a rational equation as a corrective factor to precisely describe the strain-dependent relaxation effects.
Results
The experiments revealed that (1) stress is insensitive to strain rate in arterial tissue when the loading rate is low, and (2) the rate of stress relaxation of arterial wall is highly strain dependent. The proposed model can accurately represent the experimental data. Stress–strain function derived from the combined strain energy function is able to fit the tensile experimental data with R 2 equals to 0.9995 in circumferential direction and 0.999 in longitudinal direction. Modified reduced relaxation function is able to model the strain-dependent relaxation with R 2 equals to 0.9686 in circumferential direction and 0.988 in longitudinal direction.
Conclusion
The proposed model, based on extensive biomechanical experiments, can be used for accurate simulation of arterial deformation and haptic rendering in surgical simulation. The resultant model enables stress relaxation status to be determined when subjected to different strain levels.
Similar content being viewed by others
References
Basdogan C et al (2004) Haptics in minimally invasive surgical simulation and training. IEEE Comput Graph Appl 24(2): 56–64
Chanda A, Kesavadas T (2004) Real-time volume haptic rendering of non-linear viscoelastic behavior of soft tissue through dynamic atomic unit approach. Stud Health Technol Inform 98: 49–55
Takamizawa K, Hayashi K (1987) Strain energy density function and uniform strain hypothesis for arterial mechanics. J Biomech 20(1): 7–17
Tanaka TT, Fung YC (1974) Elastic and inelastic properties of the canine aorta and their variation along the aortic tree. J Biomech 7(4): 357–370
Hayashi K et al (1981) Mechanical properties of aortas and pulmonary arteries of calves implanted with cardiac prostheses. J Biomech 14(3): 173–182
Veress AI et al (2000) Vascular mechanics of the coronary artery. Z Kardiol 89(Suppl 2): 92–100
Chuong CJ FY (1984) Compressibility and constitutive equation of arterial wall in radial compression experiments. J Biomech 17(1): 35–40
Fung YC, (1993) Biomechanics : mechanical properties of living tissues, 2nd edn. Springer, New York, xviii, 568 p
Nichols WW, O’Rourke MF, McDonald DA (2005) McDonald’s blood flow in arteries : theoretic, experimental, and clinical principles, 5th edn. Hodder Arnold, London New York; Distributed in the U.S.A. by Oxford University Press. xii, 607 p
Roach MR, Burton AC (1957) The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol 35(8): 681–690
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61(1–3): 1–48
Chuong CJ, Fung YC (1983) Three-dimensional stress distribution in arteries. J Biomech Eng 105(3): 268–274
Chuong CJ, Fung YC (1986) On residual stresses in arteries. J Biomech Eng 108(2): 189–192
Fung YC, Liu SQ, Zhou JB (1993) Remodeling of the constitutive equation while a blood vessel remodels itself under stress. J Biomech Eng 115(4B): 453–459
Humphrey JD (1999) An evaluation of pseudoelastic descriptors used in arterial mechanics. J Biomech Eng 121(2): 259–262
Chui C et al (2007) Transversely isotropic properties of porcine liver tissue: experiments and constitutive modelling. Med Biol Eng Comput 45(1): 99–106
Spirt AA, Mak AF, Wassell RP (1989) Nonlinear viscoelastic properties of articular cartilage in shear. J Orthop Res 7(1): 43–49
Park S, Ateshian GA (2006) Dynamic response of immature bovine articular cartilage in tension and compression, and nonlinear viscoelastic modeling of the tensile response. J Biomech Eng 128(4): 623–630
Javad Hazrati Marangalou FGBM (2008) Application of modified superposition model to viscoelastic behavior of periodontal ligment. J Biomed Sci Eng 1(3): 195–199
Hazrati J, Ghalichi F, Mirzakouchaki B (2008) Strain dependent stress relaxation behavior of periodonatal ligament. J Biomech 41(Suppl 1): S471
Provenzano P et al (2001) Nonlinear ligament viscoelasticity. Ann Biomed Eng 29(10): 908–914
Hingorani RV et al (2004) Nonlinear viscoelasticity in rabbit medial collateral ligament. Ann Biomed Eng 32(2): 306–312
Pena E, Pena JA, Doblare M (2008) On modelling nonlinear viscoelastic effects in ligaments. J Biomech 41(12): 2659–2666
Sarver JJ, Robinson PS, Elliott DM (2003) Methods for quasi-linear viscoelastic modeling of soft tissue: application to incremental stress-relaxation experiments. J Biomech Eng 125(5): 754–758
Drozdov AD (1998) Mechanics of viscoelastic solids. Wiley, New York, xii, 472 p
Drozdov AD (1996) A constitutive model in thermoviscoelasticity. Mech Res Commun 23(5): 543–548
He ZR, Song MS (1993) Elastic behaviors of swollen multiphase networks of SBS and SIS block copolymers. Rheol Acta 32(3): 254–262
Lally C, Reid AJ, Prendergast PJ (2004) Elastic behavior of porcine coronary artery tissue under uniaxial and equibiaxial tension. Ann Biomed Eng 32(10): 1355–1364
Lee MC, Haut RC (1989) Insensitivity of tensile failure properties of human bridging veins to strain rate: implications in biomechanics of subdural hematoma. J Biomech 22(6–7): 537–542
Lee MC, Haut RC (1992) Strain rate effects on tensile failure properties of the common carotid artery and jugular veins of ferrets. J Biomech 25(8): 925–927
Mohan D, Melvin JW (1982) Failure properties of passive human aortic tissue. I–uniaxial tension tests. J Biomech 15(11): 887–902
Mohan D, Melvin JW (1983) Failure properties of passive human aortic tissue. II–Biaxial tension tests. J Biomech 16(1): 31–44
Stemper BD, Yoganandan N, Pintar FA (2007) Mechanics of arterial subfailure with increasing loading rate. J Biomech 40(8): 1806–1812
Stemper BD, Yoganandan N, Pintar FA (2005) Methodology to study intimal failure mechanics in human internal carotid arteries. J Biomech 38(12): 2491–2496
van Dam EA et al (2008) Non-linear viscoelastic behavior of abdominal aortic aneurysm thrombus. Biomech Model Mechanobiol 7(2): 127–137
Wu SG, Lee GC (1984) On nonlinear viscoelastic properties of arterial tissue. J Biomech Eng 106(1): 42–47
Yeung ZLaK (2008) The preconditioning and stress relaxation of skin tissue. J Biomed Pharm Eng 2(1): 22–28
Tan HZ et al (1994) Human factors for the design of force-reflecting haptic interfaces. Proc ASME Dyn Syst Control Div DCS-55(1): 353–359
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, T., Chui, C.K., Yu, R.Q. et al. Quasi-linear viscoelastic modeling of arterial wall for surgical simulation. Int J CARS 6, 829–838 (2011). https://doi.org/10.1007/s11548-011-0560-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-011-0560-x