Abstract
Purpose
To determine the clinical, pathological, and radiological features, including the Vesical Imaging-Reporting and Data System (VI-RADS) score, independently correlating with muscle-invasive bladder cancer (BCa), in a multicentric national setting.
Method and Materials
Patients with BCa suspicion were offered magnetic resonance imaging (MRI) before trans-urethral resection of bladder tumor (TURBT). According to VI-RADS, a cutoff of ≥ 3 or ≥ 4 was assumed to define muscle-invasive bladder cancer (MIBC). Trans-urethral resection of the tumor (TURBT) and/or cystectomy reports were compared with preoperative VI-RADS scores to assess accuracy of MRI for discriminating between non-muscle-invasive versus MIBC. Performance was assessed by ROC curve analysis. Two univariable and multivariable logistic regression models were implemented including clinical, pathological, radiological data, and VI-RADS categories to determine the variables with an independent effect on MIBC.
Results
A final cohort of 139 patients was enrolled (median age 70 [IQR: 64, 76.5]). MRI showed sensitivity, specificity, PPV, NPV, and accuracy for MIBC diagnosis ranging from 83–93%, 80–92%, 67–81%, 93–96%, and 84–89% for the more experienced readers. The area under the curve (AUC) was 0.95 (0.91–0.99). In the multivariable logistic regression model, the VI-RADS score, using both a cutoff of 3 and 4 (P < .0001), hematuria (P = .007), tumor size (P = .013), and concomitant hydronephrosis (P = .027) were the variables correlating with a bladder cancer staged as ≥ T2. The inter-reader agreement was substantial (k = 0.814).
Conclusions
VI-RADS assessment scoring proved to be an independent predictor of muscle-invasiveness, which might implicate a shift toward a more aggressive selection approach of patients’ at high risk of MIBC, according to a novel proposed predictive pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Bladder cancer (BCa) is the 10th most diagnosed tumor in the world, and the new 5-years prevalence estimates show that 1,720,625 people are living with bladder cancer within five years of a past diagnosis, since it is a highly recurrent tumor [1]. Interestingly, BCa-related costs are caused by the hospital care and represent 5% of total health care cancer costs [2]. This is probably linked to the fact that the diagnostic pathway for bladder cancer patients has been mostly unchanged for more than 30 years, with trans-urethral resection of bladder tumor (TURBT) as the initial diagnostic and staging tool [3]. Therefore, an adequate, effective, and personalized management of this disease, from the diagnostic-staging to the therapeutic steps of the work-up, should be envisioned to bring major beneficial implications on patients’ well-being. That is why, in the last years, the role of MRI for BCa staging has represented a hot topic in the urological community. A turning point has occurred when the Vesical Imaging-Reporting and Data System (VI-RADS) was released in 2018 to improve standardization, reliability and reproducibility of MR images acquisition and reporting, to optimally differentiate non-muscle-invasive from muscle-invasive BCa (NMIBC vs MIBC) which represents the diagnostic cornerstone of the disease [4]. Since then, the VI-RADS assessment score has been validated by different groups and up to now three meta-analysis have been published showing optimal results in terms of VI-RADS diagnostic performance and inter-reader reproducibility [5,6,7,8,9,10]. The most recent meta-analyses showed a pooled weighted mean κ estimate of 0.83 (95% CI 0.78–0.88) and pooled sensitivity, specificity, and area under the curve (AUC) value of 0.77–0.90 (95% CI 0.65–0.94), 0.97–0.86 (95% CI 0.71–0.99), and 0.92–0.93 (95% CI 0.89–0.95) for VI-RADS 3–4 as the cutoff value for MIBC [6, 7]. However, while the accuracy of VI-RADS has been reasonably proven, it is not clear yet whether clinical and pathological features can influence VI-RADS categorization, and in turn whether the VI-RADS can better predict the risk of MIBC alone or rather in combined models including extra-radiological factors.
With this background, the aim of our study was to determine the clinical, radiological, and pathological features associated with muscle-invasive bladder cancer. We tested the hypothesis that multi-level variables might improve the current treatment paradigm of BCa in the pre-treatment setting.
Materials and methods
Study design and patients population
This prospective multicenter multireader observational study received formal approval from the Institutional Review Board and the Ethical Committee of each participating center. All patients were notified of the investigational nature of this study and gave their written informed consent. The study was conducted in accordance with the guidelines for good clinical practice in accordance with ethical principles as reported in the latest version of the Declaration of Helsinki. All patients with suspicion of BCa were referred to four different centers (Sapienza University of Rome [center #1], "Regina Elena" National Cancer Institute of Rome [center #2], Santa Maria della Misericordia University Hospital in Udine [center #3] and the University Hospital of Bologna [center #4]), between January 2020 and May 2021, and were offered bladder MRI. The inclusion criteria were a primary diagnosis of bladder tumor, positive urinary cytology, suspected bladder neoplasm identified by ultrasound of the urinary tract and/or cystoscopy and/or CT scan of the abdomen-pelvis. The exclusion criteria were history of prior urinary tract neoplasms, impossibility of achieving appropriate bladder distension, concomitant diagnosis of carcinoma in situ (CIS), no detectable lesion on MRI and any contraindication to MRI (low renal function, MR unsafe medical devices etc.) and to spinal and general anesthesia. No previous study was conducted on the same population cohort.
MR imaging acquisition protocol and image analysis
MRI of the pelvis was performed using a 3 Tesla magnet (Discovery MR750, GE Healthcare, Milwaukee, WI) (center #1 and #2), using a 3 Tesla magnet (Achieva, Philips) (center #3), both equipped with a 32-channel phased-array body coil, and a 1.5 Tesla scanner (Signa HDxt; GE Healthcare, Milwaukee, WI) equipped with an 8-channel phased-array surface coil (center #4). The MR imaging protocol was in accordance with the original VI-RADS document [4]. Patients were administered an intramuscular antispasmodic (n-butyl-scopolamine 20 mg) agent when necessary to lower bladder wall motion and were instructed to drink 500–1000 ml of water 30 min before the examination to obtain adequate bladder distension. A summary Supplementary Table 1 details the MRI acquisition parameters.
Image analysis was performed independently by two radiologists in each center, one more experienced (ME) and one less experienced (LE), respectively, with 15 and 4 (center #1 and #2), 15 and 5 (center #3) and 10 and 5 years of experience (center #4), respectively. Cumulative reading experience for less experienced readers ranged from 20 to 80 cases. MRIs of patients enrolled in center #2 were acquired and analyzed by radiologists from center #1. Each reader analyzed images blindly to clinical data, following the evaluation algorithm of the VI-RADS system. VI-RADS cutoff scores of 3 or greater and 4 or greater were used to define muscle invasiveness in our analysis. For patients with more than one lesion, only the lesion with the highest VI-RADS score was used.
Standard of reference (TURBT, Re-TURBT and radical cystectomy)
All enrolled patients underwent primary conventional TURBT (white light) within 6 weeks after bladder MRI. All endoscopic resection procedures were performed by a single operator designated for each enrollment center. Each endoscopic resection procedure was performed adhering to the dictates of the EAU guidelines [11]. Patients who were candidates for Re-TURBT underwent secondary resection of the tumor within 2–6 weeks of TURBT. The following cases were considered candidates for Re-TURBT, according to EAU guidelines: incomplete endoscopic resection at first TURBT, T1 patients histologically established by TURBT, and patients without detrusor muscle inclusion in the histologic specimen of the first TURBT except for low-grade Ta. All Re-TURBTs were performed by the same operator who previously performed the TURBT using conventional resection technique with monopolar or bipolar current. The site of the previous resection area identified as corresponding to the lesion characterized by the highest VI-RADS score was separately sampled and analyzed. All pathology reports performed by a dedicated pathologist with more than 10 years of experience in the field (TURBT, Re-TURBT and RC) defined staging of the disease according to the TNM classification [12] and the WHO 2004 classification (grading system) to determine the degree of cellular anaplasia [13]. Standard of reference was considered the pathology report of the primary and/or secondary TURBT, or RC when performed.
Statistical analysis
The performance of MRI was assessed by means of receiver operating characteristic curve analysis for both the more and less experienced readers. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated to assess the performance of VI-RADS scoring by each reader. In addition, a per-sequence receiver operating characteristic curve analysis was performed. Inter-reader agreement analysis between the more and less experienced readers was performed with Cohen’ K statistics for overall VI-RADS assessment and on a per-sequence basis, to investigate scoring variability. Correlation between patient demographics, clinical characteristics and VI-RADS was analyzed using Pearson’s Chi-squared test. The normality of the variables was tested. Two univariable analyses were performed: the first included clinical/pathological features (patient age and sex, history of smoking [number of cigarettes, years of smoking], Charlson Comorbidity Index [CCI], body mass index [BMI], presence of hematuria [micro- and macrohematuria], number of foci at TURBT, grading [low/high and G1/2/3]), and the overall VI-RADS categories; the second included VI-RADS and all radiological features (lesion size, the presence of tumor peduncle and inner layer, hydronephrosis, and suspicious lymph nodes). For both analyses, a multivariable logistic regression model including only significative variables at univariable analysis adjusted for age and sex was implemented to determine the features that had independent effect on MIBC detection. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 28. All tests were two-sided, and statistical significance was set at P < 0 0.05.
Results
Patient characteristics
Overall, 151 consecutive patients were enrolled and underwent MRI (72 patients from center #1, 51 patients from center #2, 16 from center #3 and 12 from center #4). Out of these patients, 12 (8%) had at least one exclusion criteria and were excluded. Thus, 139 patients were included in the final patient sample. The median ages were 70 years (interquartile range, 64–76.5 years) among 103 (74%) males and 36 (26%) female patients. Patient characteristics are shown in Table 1, according to invasiveness of the disease.
Diagnostic performance of VI-RADS assessment
The performance of VI-RADS assessment in detecting muscle-invasive bladder cancer using VI-RADS 3 and 4 as cutoff for muscle invasion detection is presented in detail in Table 2. The AUCs for detecting MIBC of more and less experienced readers were 0.95 (95% CI 0.91, 0.99) and 0.93 (95% CI 0.88, 0,98), respectively. Figure 1 shows the receiver operating characteristic curves of overall VI-RADS assessment for both more and less experienced readers. On a per-sequence analysis, overall VI-RADS showed the highest AUC compared to the single sequences (T2WI: 0.94 [95% CI 0.90, 0.98]; DWI: 0.93 [95% CI 0.87, 0.98]; DCE: 0.94 [95% CI 0.90, 0.98]) for both groups of readers; details are shown in Supplementary Table 2.
Inter-reader agreement between the more and less experienced radiologists using k statistics was 0,814 (P < 0.001) for overall VI-RADS assessment. Supplementary Tables 2 and 3 summarize the k statistics for each MRI sequence and overall VI-RADS assessment. Figure 2 shows a case in which there was no agreement between the readers.
Case example of a 66-years-old female that was incorrectly scored as a VI-RADS 4 by the less experienced readers, but correctly scored as VI-RADS 2 by the more experienced readers. (a–b–c) Axial, sagittal and coronal T2-weighted imaging showing a pedunculated bladder tumor (20 mm) at the right lateral wall; (d–e) diffusion-weighted imaging and ADC map showing the “inchworm sign”, typical of non-muscle-invasive bladder cancer; (f) dynamic contrast-enhanced MRI showing integrity of the muscularis propria layer and enhancement of the “inner layer” (arrow). The lesion should be classified as VI-RADS 2. VI-RADS, Vesical Imaging-Reporting and Data System; ADC, apparent diffusion coefficient
Univariable and multivariable analysis for determining the clinical, radiological, and pathological features associated with muscle-invasive bladder cancer
On univariable analysis performed using clinical features, pathology data and VI-RADS, the number of cigarettes smoked per day (< 15 or ≥ 15) (P = 0.02), concomitant hematuria (either micro or macro) (P = 0.002), the number of TURBT foci (1 or > 1) (P = 0.002), the WHO scoring grading (low or high grade) (P < 0.0001), the 1973 grading (G1 vs. G2 vs. G3) (P = 0.001), and the VI-RADS assessment using both cutoff 3 and 4 (P < 0.0001) correlated with MIBC. In the multivariable logistic regression model, the variables showing independent correlation with MIBC were concomitant hematuria (odds ratio [OR]: 11.0 [95% CI 1.93–62.73]; P = 0.007) and the VI-RADS assessment with a cutoff of 3 or greater (OR: 55.15 [95% CI 9.17, 331.54]; P < 0.0001). Using a VI-RADS cutoff of 4, the only independent variable was the VI-RADS scoring itself (OR: 39.27 [95% CI 8.13, 189.80]; P < 0.0001).
On univariable analysis performed using only radiological features, MIBC correlated with the VI-RADS assessment using both cutoff 3 and 4 (P < 0.0001), with the lesion size (< 2.5 cm or ≥ 2.5 cm) (P < 0.0001), with the presence of the vascular peduncle (P < 0.0001) and the inner layer (P = 0.001), and with the presence of suspicious lymph nodes (P < 0.0001). In the multivariable logistic regression model, a VI-RADS score confirmed to be independent predictor of MIBC using both cutoff of 3 and 4 (cutoff of 3: OR: 15.98 [95% CI 3.90, 65.94]; P < 0.0001; cutoff of 4: OR: 22.16 [95% CI 6.12, 80.27]; P < 0.0001). In addition, using both cutoff, lesion size showed independent correlation (cutoff of 3: OR: 4.32 [95% CI 1.36, 13.65]; P = 0.013; cutoff of 4: OR: 4.43 [95% CI 1.27, 15.42]; P = 0.02). Instead, only when applying a VI-RADS a cutoff of 4, also the concomitant presence of hydronephrosis was a predictor of MIBC (OR: 19.07 [95% CI 1.40, 259.72]; P = 0.02). Age and sex, smoking history alone and total years of smoking, CCI, and BMI were not predictive of MIBC. Tables 3 and 4 show the results of both univariable and multivariable analyses.
Discussion
In recent years, bladder MRI and the VI-RADS assessment score have gained interests in the scientific community since they represent the imaging tools that provide the best diagnostic performance for bladder cancer local staging, accurately differentiating NMIBC from MIBC.
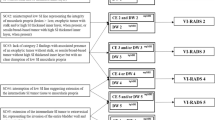
In our analysis, the VI-RADS score confirmed the high diagnostic performance already demonstrated in many other investigations [14,15,16,17,18,19,20]. Indeed, we found that assessing bladder MRI using the VI-RADS score provided a sensitivity ranging 83–93%, specificity 80–92%, PPV 67–81%, NPV 93–96%, and accuracy of 84–89% for the more experienced readers; the less experienced readers showed a sensitivity ranging 83–95%, specificity 73–86%, PPV 60–71%, NPV 92–97%, and accuracy 80–85%. The AUC was 0.95 (0.91–0.99) for the more experienced readers and 0.93 (0.88–0.98) for the less experienced readers. The inter-reader agreement was substantial (k = 0.814). These results are in line with what found in the other two multicenter multireader studies. Recently, Metwally et al. prospectively enrolled 331 patients and showed that the VI-RADS score is reliable (k = 0.93) among four readers, and valid (sensitivity of 84%, and specificity of 92%) when using a cutoff of 3 for defining muscularis propria invasion [21]. Also, Ueno et al. showed a moderate to substantial agreement (k = 0.55–0.75) between seven radiologists (experienced and inexperienced readers), with a pooled AUC of 0.88 (0.82, 0.91) and 0.84 (0.83, 0.85) for experienced and inexperienced readers, respectively [22]. However, the primary aim of this study was to provide evidence on this topic beyond validating the score itself. Indeed, we pursued to identify, among clinical, pathological, and radiological variables, the factor independently correlating with muscle-invasive bladder cancer. The logistic regression models showed how VI-RADS score, using both a cutoff of 3 and 4 (P < 0.0001), hematuria (P = 0.007), tumor size (P = 0.013), and concomitant hydronephrosis (P = 0.027) were the variables correlating with a bladder cancer staged as ≥ T2 at either TURBT, re-TURBT, or radical cystectomy. In the past several decades, many different prognostic tools have been proposed in diverse forms, such as risk grouping/stratification [23, 24], artificial neural networks [25, 26], neurofuzzy models [27, 28], and probability nomogram [29, 30]. Often, such models were developed to predict either NMIBC recurrence, response to therapy, or overall survival [31]. However, there are no tools nowadays that efficaciously incorporate clinic-pathological data to MRI biomarkers such as the VI-RADS score, tumor size, and the presence of hydronephrosis, to preoperatively predict MIBC and to envision a tailored diagnostic pathway in which patients presenting with such characteristics would be directed to sampling TUR for confirmatory pathology, avoiding the complications related to deep TURBT, and finally to identify high-risk patients who should be directed to early radical treatment. In this regards, VI-RADS score 5 has been previously correlated with a significant delay in time to cystectomy (OR 2.81, 95% CI 1.20, 6.62). [32]. This approach might overcome the issues encountered in the first and sole clinical trial assessing whether TURBT can be replaced by MRI; indeed, preliminary results from the BladderPath trial suggested that MRI causes high rate of false-positivity; however, definitive conclusions can be made only once the enrollment of the final sample size will be done [33]. The proposed pathway might facilitate the shared decision process, potentially improving clinical outcomes of patients affected with BCa (depicted in detail in Fig. 3).
Proposed predictive pathway based on the clinical and radiological variable showing independent correlation to MIBC. US, ultrasound; CT, computerized tomography; VI-RADS, Vesical Imaging-Reporting and Data System; TURBT, trans-urethral resection of bladder tumor; BCG, bacillus Calmette-Guérin; TUR, trans-urethral resection; RC, radical cystectomy; NAC, neoadjuvant chemotherapy
Our analyses are based on data from four institutions, even though MRIs were acquired and interpreted in three centers, without a homogeneous patients’ enrollment, thus limiting the immediate clinical application of our findings. In addition, MRI does not possess the sufficient spatial resolution to diagnose carcinoma in situ, which are per-definition high-risk cases. Also, no conclusion can be drawn on long-term prognosis since no analysis was performed on patients’ overall survival, nor recurrence rates due to limited follow-up data. Finally, the proposed predictive pathway lacks internal and external validation, and assessment of its clinical utility is warranted before it can be incorporated into routine clinical practice. Further research will focus on the independent validation of the findings.
Conclusion
The VI-RADS assessment score was confirmed to be an accurate preoperative tool in predicting bladder cancer invasiveness, in a multicentric setting where MRI acquisition- and reporting-related biases can be overcome. Also, if considered as an MRI biomarker, and associated with clinical data and additional imaging features, VI-RADS can be incorporated into a predictive diagnostic pathway to perform a tailored patients’ selection to therapy, with the aim of preventing disease understaging and improving clinical outcomes of patients affected with bladder cancer.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA A Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA (2016) Economic burden of bladder cancer across the European Union. Eur Urol 69:438–447. https://doi.org/10.1016/j.eururo.2015.10.024
Mostafid H, Babjuk M, Bochner B et al (2020) Transurethral resection of bladder tumour: the neglected procedure in the technology race in bladder cancer. Eur Urol 77:669–670. https://doi.org/10.1016/j.eururo.2020.03.005
Panebianco V, Narumi Y, Altun E et al (2018) Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (vesical imaging-reporting and data system). Eur Urol 74:294–306. https://doi.org/10.1016/j.eururo.2018.04.029
Woo S, Panebianco V, Narumi Y et al (2020) Diagnostic performance of vesical imaging reporting and data system for the prediction of muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur Urol Oncol. https://doi.org/10.1016/j.euo.2020.02.007
Del Giudice F, Pecoraro M, Vargas HA et al (2020) Systematic review and meta-analysis of vesical imaging-reporting and data system (VI-RADS) inter-observer reliability: an added value for muscle invasive bladder cancer detection. Cancers (Basel). https://doi.org/10.3390/cancers12102994
Luo C, Huang B, Wu Y et al (2020) Use of vesical imaging-reporting and data system (VI-RADS) for detecting the muscle invasion of bladder cancer: a diagnostic meta-analysis. Eur Radiol. https://doi.org/10.1007/s00330-020-06802-z
Panebianco V, Giganti F, Kitzing YX et al (2018) An update of pitfalls in prostate mpMRI: a practical approach through the lens of PI-RADS v. 2 guidelines. Insights Imaging 9:87–101. https://doi.org/10.1007/s13244-017-0578-x
Panebianco V, Sciarra A, Marcantonio A et al (2012) Conventional imaging and multiparametric magnetic resonance (MRI, MRS, DWI, MRP) in the diagnosis of prostate cancer. Q J Nucl Med Mol Imaging 56:331–342
Sciarra A, Panebianco V, Cattarino S et al (2012) Multiparametric magnetic resonance imaging of the prostate can improve the predictive value of the urinary prostate cancer antigen 3 test in patients with elevated prostate-specific antigen levels and a previous negative biopsy. BJU Int 110:1661–1665. https://doi.org/10.1111/j.1464-410X.2012.11146.x
M Babjuk, M Burger, E Compérat, P Gontero, AH Mostafid, & J Palou (2019) EAU guidelines on non-muscle-invasive bladder cancer.
Brierley J, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumours, 8th edn. Wiley, Chichester
Moch H, Cubilla AL, Humphrey PA et al (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol 70:93–105. https://doi.org/10.1016/j.eururo.2016.02.029
Wang H, Luo C, Zhang F et al (2019) Multiparametric MRI for bladder cancer: validation of VI-RADS for the detection of detrusor muscle invasion. Radiology 291:668–674. https://doi.org/10.1148/radiol.2019182506
Kim SH (2019) Validation of vesical imaging reporting and data system for assessing muscle invasion in bladder tumor. Abdom Radiol. https://doi.org/10.1007/s00261-019-02190-1
Wang Z, Shang Y, Luan T et al (2020) Evaluation of the value of the VI-RADS scoring system in assessing muscle infiltration by bladder cancer. Cancer Imaging 20:26. https://doi.org/10.1186/s40644-020-00304-3
Liu S, Feijia Xu, Tianyuan Xu, Yan Y, Yao X, Tang G (2020) Evaluation of vesical imaging-reporting and data system (VI-RADS) scoring system in predicting muscle invasion of bladder cancer. Transl Androl Urol 9(2):445–451. https://doi.org/10.21037/tau.2020.02.16
Cao B, Li Q, Xu P et al (2022) Preliminary exploration of the application of vesical imaging-reporting and data system ( vi-rads ) in post-treatment patients with bladder cancer: a prospective single-center study. Magn Reson Imaging 55:275–286. https://doi.org/10.1002/jmri.27807
Del Giudice F, Barchetti G, De Berardinis E et al (2020) Prospective assessment of vesical imaging reporting and data system (VI-RADS) and Its clinical impact on the management of high-risk non–muscle-invasive bladder cancer patients candidate for repeated transurethral resection. Eur Urol 77:101–109. https://doi.org/10.1016/j.eururo.2019.09.029
Barchetti G, Simone G, Ceravolo I et al (2019) Multiparametric MRI of the bladder: inter-observer agreement and accuracy with the vesical imaging-reporting and data system (VI-RADS) at a single reference center. Eur Radiol 29:5498–5506. https://doi.org/10.1007/s00330-019-06117-8
Metwally MI, Zeed NA, Hamed EM et al (2021) The validity, reliability, and reviewer acceptance of VI-RADS in assessing muscle invasion by bladder cancer: a multicenter prospective study. Eur Radiol. https://doi.org/10.1007/s00330-021-07765-5
Ueno Y, Tamada T, Takeuchi M et al (2021) VI-RADS: multiinstitutional multireader diagnostic accuracy and interobserver agreement study. AJR Am J Roentgenol 216:1257–1266. https://doi.org/10.2214/AJR.20.23604
Parmar MKB, Freedman LS, Hargreave TB, Tolley DA (1989) Prognostic factors for recurrence and followup policies in the treatment of superficial bladder cancer: report from the british medical research council subgroup on superficial bladder cancer (urological cancer working party). J Urol 142:284–288. https://doi.org/10.1016/S0022-5347(17)38731-1
Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J et al (2000) Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 164:680–684. https://doi.org/10.1016/S0022-5347(05)67280-1
Fujikawa K, Matsui Y, Kobayashi T et al (2003) Predicting disease outcome of non-invasive transitional cell carcinoma of the urinary bladder using an artificial neural network model: Results of patient follow-up for 15 years or longer. Int J Urol 10:149–152. https://doi.org/10.1046/j.1442-2042.2003.00589.x
Qureshi KN, Naguib RN, Hamdy FC et al (2000) Neural network analysis of clinicopathological and molecular markers in bladder cancer. J Urol 163:630–633
Catto JWF, Abbod MF, Linkens DA, Hamdy FC (2006) Neuro-fuzzy modeling: an accurate and interpretable method for predicting bladder cancer progression. J Urol 175:474–479. https://doi.org/10.1016/S0022-5347(05)00246-6
Catto JWF, Linkens DA, Abbod MF et al (2003) Artificial intelligence in predicting bladder cancer outcome: a comparison of neuro-fuzzy modeling and artificial neural networks. Clin Cancer Res 9:4172–4177
Ali-El-Dein B, Sooriakumaran P, Trinh Q-D et al (2013) Construction of predictive models for recurrence and progression in >1000 patients with non-muscle-invasive bladder cancer (NMIBC) from a single centre. BJU Int 111:E331-341. https://doi.org/10.1111/bju.12026
Kluth LA, Black PC, Bochner BH et al (2015) Prognostic and prediction tools in bladder cancer: a comprehensive review of the literature. Eur Urol 68:238–253. https://doi.org/10.1016/j.eururo.2015.01.032
Cambier S, Sylvester RJ, Collette L et al (2016) EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non–muscle-invasive stage Ta–T1 urothelial bladder cancer patients treated with 1–3 Years of maintenance bacillus calmette-guérin. Eur Urol 69:60–69. https://doi.org/10.1016/j.eururo.2015.06.045
Del Giudice F, Leonardo C, Simone G et al (2020) Preoperative detection of VI-RADS (vesical imaging-reporting and data system) score 5 reliably identifies extravesical extension of urothelial carcinoma of the urinary bladder and predicts significant delayed time-to-cystectomy: time to reconsider the nee. BJU Int. https://doi.org/10.1111/bju.15188
Bryan RT, Liu W, Pirrie SJ et al (2021) Comparing an imaging-guided pathway with the standard pathway for staging muscle-invasive bladder cancer: preliminary data from the bladderpath study. Eur Urol 80:12–15. https://doi.org/10.1016/j.eururo.2021.02.021
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Valeria Panebianco supervised the project and edited the final version of the manuscript. Material preparation, data collection, and analysis were performed by Marco Bicchetti, Giuseppe Simone, Rossano Girometti, Martina Pecoraro, Francesco Del Giudice, Simone Flammia, and Caterina Gaudiano. The first draft of the manuscript was written by Marco Bicchetti and Gianluca Giannarini, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Approval was obtained from the ethics committee of Sapienza University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bicchetti, M., Simone, G., Giannarini, G. et al. A novel pathway to detect muscle-invasive bladder cancer based on integrated clinical features and VI-RADS score on MRI: results of a prospective multicenter study. Radiol med 127, 881–890 (2022). https://doi.org/10.1007/s11547-022-01513-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-022-01513-5