Abstract
Aims
The aim of the study was to predict and assess treatment response by histogram analysis of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to patients with locally advanced esophageal squamous cell carcinoma receiving chemoradiotherapy (CRT).
Materials and methods
Seventy-two patients with locally advanced esophageal squamous cell carcinoma who underwent DCE-MRI before and after chemoradiotherapy were enrolled and divided into the complete response (CR) group and the non-CR group based on RECIST. The histogram parameters (10th percentile, 90th percentile, median, mean, standard deviation, skewness, and kurtosis) of pre-CRT and post-CRT were compared using a paired Student’s t test in the CR and non-CR groups, respectively. The histogram parameter differences between the CR and the non-CR groups were compared using an unpaired Student’s t test. A receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic performance.
Results
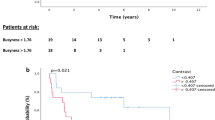
The histogram parameters of Ktrans values were observed to have significantly decreased after chemoradiotherapy in the CR group. The CR responders showed significantly higher median, mean, and 10th and 90th percentile of pre-Ktrans values than those of the non-CR group. The histogram analysis indicated the decreased heterogeneity in the CR group after CRT. Esophageal cancer with higher pre-Ktrans and lower post-Ktrans values indicated a good treatment response to CRT. Pre-Ktrans-10th showed the best diagnostic performance in predicting the chemoradiotherapy response.
Conclusions
The histogram parameters of Ktrans are useful in the assessment and prediction of the chemoradiotherapy response in patients with advanced esophageal squamous cell carcinoma. DCE-MRI could serve as an adjunctive imaging technique for treatment planning.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Heethuis SE, van Rossum PSN, Lips IM et al (2016) Dynamic contrast-enhanced MRI for treatment response assessment in patients with oesophageal cancer receiving neoadjuvant chemoradiotherapy. Radiother Oncol 120:128–135
Kwee Robert M, Dik Alexander K, Sosef Meindert N et al (2014) Interobserver reproducibility of diffusion-weighted MRI in monitoring tumor response to neoadjuvant therapy in esophageal cancer. PLoS ONE 9:e92211
Tao CJ, Lin G, Xu YP, Mao WM (2015) Predicting the response of neoadjuvant therapy for patients with esophageal carcinoma: an in-depth literature review. J Cancer 6:1179–1186
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Allum WH, Stenning SP, Bancewicz J et al (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27:5062–5067
Oberholzer K, Pohlmann A, Schreiber W et al (2008) Assessment of tumor microcirculation with dynamic contrast-enhanced MRI in patients with esophageal cancer: initial experience. J Magn Reson Imaging 27:1296–1301
Suo S, Zhang K, Cao M et al (2016) Characterization of breast masses as benign or malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magn Reson Imaging 43:894–902
Chang YC, Huang CS, Liu YJ et al (2004) Angiogenic response of locally advanced breast cancer to neoadjuvant chemotherapy evaluated with parametric histogram from dynamic contrast-enhanced MRI. Phys Med Biol 49:3593–3602
Liu S, Zhen F, Sun N et al (2016) Apparent diffusion coefficient values detected by diffusion-weighted imaging in the prognosis of patients with locally advanced esophageal squamous cell carcinoma receiving chemoradiation. Onco Targets Ther 9:5791–5796
Xu XQ, Hu H, Su GY et al (2016) Utility of histogram analysis of ADC maps for differentiating orbital tumors. Diagn Interv Radiol 22:161–167
Heo SH, Shin SS, Kim JW et al (2013) Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol 14:616–625
Lerant G, Sarkozy P, Takacsi-Nagy Z et al (2015) Dynamic contrast-enhanced MRI parameters as biomarkers in assessing head and neck lesions after chemoradiotherapy using a wide-bore 3 Tesla scanner. Pathol Oncol Res 21:1–9
Lei J, Han Q, Zhu S et al (2015) Assessment of esophageal carcinoma undergoing concurrent chemoradiotherapy with quantitative dynamic contrast-enhanced magnetic resonance imaging. Oncol Lett 10:3607–3612
Kim JH, Kim CK, Park BK et al (2012) Dynamic contrast-enhanced 3-T MR imaging in cervical cancer before and after concurrent chemoradiotherapy. Eur Radiol 22:2533–2599
Intven M, Reerink O, Philippens ME (2015) Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neoadjuvant chemoradiation. J Magn Reson Imaging 41:1646–1653
Chikui T, Kitamoto E, Kawano S et al (2012) Pharmacokinetic analysis based on dynamic contrast-enhanced MRI for evaluating tumor response to preoperative therapy for oral cancer. J Magn Reson Imaging 36:589–597
Just N (2014) Improving tumor heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213
Murayama K, Nishiyama Y, Hirose Y et al (2018) Differentiating between central nervous system lymphoma and high-grade glioma using dynamic susceptibility contrast and dynamic contrast-enhanced MR imaging with histogram analysis. Magn Reson Med Sci 17:42–49
Jackson A, O’Connor JP, Parker GJ, Jayson GC (2007) Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res 13:3449–3459
Wu CJ, Wang Q, Li H et al (2015) DWI-associated entire-tumor histogram analysis for the differentiation of low-grade prostate cancer from intermediate–high-grade prostate cancer. Abdom Imaging 40:3214–3221
Wang HY, Su ZH, Xu X et al (2016) Dynamic contrast-enhanced MR imaging in renal cell carcinoma: reproducibility of histogram analysis on pharmacokinetic parameters. Sci Rep 6:29146
Li ZW, Ai T, Hu YQ et al (2017) Application of whole-lesion histogram analysis of pharmacokinetic parameters in dynamic contrast-enhanced MRI of breast lesions with the CAIPIRINHA-Dixon-TWIST-VIBE technique. J Magn Reson Imaging 47:91–96
Davnall F, Yip Connie SP, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
Peng SL, Chen CF, Liu HL et al (2013) Analysis of parametric histogram from dynamic contrast-enhanced MRI: application in evaluating brain tumor response to radiotherapy. NMR Biomed 26:443–450
Hector SJ, Wagner M, Bane O et al (2017) Quantification of hepatocellular carcinoma heterogeneity with multiparametric magnetic resonance imaging. Sci Rep 7:2452
Meng J, Zhu LJ, Zhu L et al (2017) Whole-lesion ADC histogram and texture analysis in predicting recurrence of cervical cancer treated with CCRT. Oncotarget 8:92442–92453
Park M, Kim J, Choi YS et al (2016) Application of dynamic contrast-enhanced MRI parameters for differentiating squamous cell carcinoma and malignant lymphoma of the oropharynx. Am J Roentgenol 206:401–407
Falk A, Fahlström M, Rostruo E et al (2014) Discrimination between glioma grades II and III in suspected low-grade gliomas using dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach. Neuroradiology 56:1031–1038
Rosenkrantz AB, Obele C, Rusinek H et al (2015) Whole-lesion diffusion metrics for assessment of bladder cancer aggressiveness. Abdom Imaging 40:327–332
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
King AD, Thoeny HC (2016) Functional MRI for the prediction of treatment response in head and neck squamous cell carcinoma: potential and limitations. Cancer Imaging 16:23
Kim SH, Lee JM, Gupta SN et al (2014) Dynamic contrast-enhanced MRI to evaluate the therapeutic response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Magn Reson Imaging 40:730–737
de Lussanet QG, Backes WH, Griffioen AW et al (2005) Dynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancer. Int J Radiat Oncol Biol Phys 63:1309–1315
Zahra MA, Hollingsworth KG, Sala E et al (2007) Dynamic contrast-enhanced MRI as a predictor of tumor response to radiotherapy. Lancet Oncol 8:63–74
Lim JS, Kim D, Baek SE et al (2012) Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 22:1693–1700
Bollschweiler E, Hölscher AH, Schmidt M et al (2015) Neoadjuvant treatment for advanced esophageal cancer: response assessment before surgery and how to predict response to chemoradiation before starting treatment. Chin J Cancer Res 27:221–230
Lee HY, Kim N, Goo JM et al (2016) Perfusion parameters as potential imaging biomarkers for the early prediction of radiotherapy response in a rat tumor model. Diagn Interv Radiol 22:231–240
Cooper RA, Carrington BM, Loncaster JA et al (2000) Tumor oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol 57:53–59
Crokart N, Radermacher K, Jordan BF et al (2005) Tumor radiosensitization by anti-inflammatory drugs: evidence for a new mechanism involving the oxygen effect. Cancer Res 65:7911–7916
Jordan BF, Runquist M, Raghunand N et al (2005) The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res 11:529–536
Pham TT, Liney GP, Wong K, Barton MB (2017) Functional MRI for quantitative treatment response prediction in locally advanced rectal cancer. Br J Radiol 90:20151078
Chawla S, Kim S, Dougherty L et al (2013) Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. Am J Roentgenol 200:35–43
Lin M, Tian MM, Zhang WP et al (2016) Predictive values of diffusion-weighted imaging and perfusion-weighted imaging in evaluating the efficacy of transcatheter arterial chemoembolization for hepatocellular carcinoma. Onco Targets Ther 9:7029–7037
Funding
This study was not funded by any financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, NN., Ge, XL., Liu, XS. et al. Histogram analysis of DCE-MRI for chemoradiotherapy response evaluation in locally advanced esophageal squamous cell carcinoma. Radiol med 125, 165–176 (2020). https://doi.org/10.1007/s11547-019-01081-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-019-01081-1