Abstract
Purpose
To retrospectively evaluate the value of whole-lesion histogram analysis of apparent diffusion coefficient (ADC) maps in differentiating between lymphoma and metastatic squamous cell carcinoma (SCC) of unknown clinical primary in neck nodes.
Methods
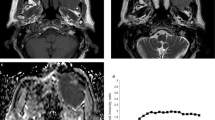
A total of 39 patients, 20 affected by lymphoma and 19 affected by metastatic non-nasopharyngeal SCC, were included in this retrospective study. All patients underwent MR imaging with a 1.5 T scanner system, including diffusion-weighted imaging (DWI) with three different b values (b = 0, 500 and 800 s/mm2). The entire tumor volume was manually delineated on the ADC maps, using the T2-weighted images and DWIs with b = 800 s/mm2 as a guide to the lesion location. The Mann–Whitney rank-sum test for independent samples was performed to compare the histogram parameters of patients with lymphoma and SCC.
Results
The SCCs showed significantly higher median ADC (ADCmedian) and mean ADC (ADCmean) values, compared to lymphomas (p < 0.001), while they exhibited lower kurtosis and skewness without reaching significance (p = 0.066 and 0.148, respectively). The ADCmean and ADCmedian had the best discriminative powers for differentiating lymphoma and SCC, with an area under the curve of 87% and 85%, respectively. The optimal cutoff values for ADCmean and ADCmedian as predictors for lymphoma were ≤ 0.83 × 10−3 mm2/s and ≤ 0.73 × 10−3 mm2/s, respectively.
Conclusions
The whole-lesion ADC histogram analysis of cervical lymphadenopathy may help to discriminate lymphomas from non-nasopharyngeal SCC in patients with unknown clinical primary tumor.





Similar content being viewed by others
References
Strojan P, Ferlito A, Langendijk JA et al (2013) Contemporary management of lymph node metastases from an unknown primary to the neck: II. A review of therapeutic options. Head Neck 35:286–293
Rodel RM, Matthias C, Blomeyer BD, Wolff HA, Jung K, Christiansen H (2009) Impact of distant metastasis in patients with cervical lymph node metastases from cancer of an unknown primary site. Ann Otol Rhinol Laryngol 118:662–669
Waltonen JD, Ozer E, Hall NC, Schuller DE, Agrawal A (2009) Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg 135:1024–1029
Padovani D, Aimoni C, Zucchetta P, Paluzzi A, Pastore A (2009) 18-FDG PET in the diagnosis of laterocervical metastases from occult carcinoma. Eur Arch Otorhinolaryngol 266:267–271
Galer CE, Kies MS (2008) Evaluation and management of the unknown primary carcinoma of the head and neck. J Natl Compr Cancer Netw 6:1068–1075
Adelstein D, Gillison ML, Pfister DG, Spencer S, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Eisele DW, Fenton M, Foote RL, Gilbert J, Haddad RI, Hicks WL Jr, Hitchcock YJ, Jimeno A, Leizman D, Lydiatt WM, Maghami E, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rocco J, Rodriguez CP, Shah JP, Weber RS, Witek M, Worden F, Yom SS, Zhen W, Burns JL, Darlow SD (2017) NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Cancer Netw 15:761–770
Herd MK, Woods M, Anand R, Habib A, Brennan PA (2012) Lymphoma presenting in the neck: current concepts in diagnosis. Br J Oral Maxillofac Surg 50:309–313
Wang YJ, Xu XQ, Hu H, Su GY, Shen J, Shi HB, Wu FY (2018) Histogram analysis of apparent diffusion coefficient maps for the differentiation between lymphoma and metastatic lymph nodes of squamous cell carcinoma in head and neck region. Acta Radiol 59:672–680
Park M, Kim J, Choi YS, Lee SK, Koh YW, Kim SH, Choi EC (2016) Application of dynamic contrast-enhanced MRI parameters for differentiating squamous cell carcinoma and malignant lymphoma of the oropharynx. AJR Am J Roentgenol 206:401–407
Maeda M, Kato H, Sakuma H, Maier SE, Takeda K (2005) Usefulness of the apparent diffusion coefficient in line scan diffusion-weighted imaging for distinguishing between squamous cell carcinomas and malignant lymphomas of the head and neck. AJNR Am J Neuroradiol 26:1186–1192
Sumi M, Sakihama N, Sumi T, Morikawa M, Uetani M, Kabasawa H, Shigeno K, Hayashi K, Takahashi H, Nakamura T (2003) Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol 24:1627–1634
King AD, Ahuja AT, Yeung DK, Fong DK, Lee YY, Lei KI, Tse GM (2007) Malignant cervical lymphadenopathy: diagnostic accuracy of diffusion-weighted MR imaging. Radiology 245:806–813
Ichikawa Y, Sumi M, Sasaki M, Sumi T, Nakamura T (2012) Efficacy of diffusion-weighted imaging for the differentiation between lymphomas and carcinomas of the nasopharynx and oropharynx: correlations of apparent diffusion coefficients and histologic features. AJNR Am J Neuroradiol 33:761–766
Zhang Y, Chen J, Shen J, Zhong J, Ye R, Liang B (2013) Apparent diffusion coefficient values of necrotic and solid portion of lymph nodes: differential diagnostic value in cervical lymphadenopathy. Clin Radiol 68:224–231
Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, Hammoud DA, Rustin GJ, Taouli B, Choyke PL (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
Jansen JF, Parra C, Lu Y, Shukla-Dave A (2016) Evaluation of head and neck tumors with functional MR imaging. Magn Reson Imaging Clin N Am 24:123–133
Taouli B, Beer AJ, Chenevert T, Collins D, Lehman C, Matos C, Padhani AR, Rosenkrantz AB, Shukla-Dave A, Sigmund E, Tanenbaum L, Thoeny H, Thomassin-Naggara I, Barbieri S, Corcuera-Solano I, Orton M, Partridge SC, Koh DM (2016) Diffusion-weighted imaging outside the brain: consensus statement from an ISMRM-sponsored workshop. J Magn Reson Imaging 44:521–540
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213
Xu XQ, Hu H, Su GY, Liu H, Hong XN, Shi HB, Wu FY (2016) Utility of histogram analysis of ADC maps for differentiating orbital tumors. Diagn Interv Radiol 22:161–167
Ahn SJ, Choi SH, Kim YJ, Kim KG, Sohn CH, Han MH, Chang KH, Min HS (2012) Histogram analysis of apparent diffusion coefficient map of standard and high B-value diffusion MR imaging in head and neck squamous cell carcinoma: a correlation study with histological grade. Acad Radiol 19:1233–1240
Srinivasan A, Chenevert TL, Dwamena BA, Eisbruch A, Watcharotone K, Myles JD, Mukherji SK (2012) Utility of pretreatment mean apparent diffusion coefficient and apparent diffusion coefficient histograms in prediction of outcome to chemoradiation in head and neck squamous cell carcinoma. J Comput Assist Tomogr 36:131–137
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323–1341
Weber AL, Rahemtullah A, Ferry JA (2003) Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin N Am 13:371–392
Pekcevik Y, Cukurova I, Arslan IB (2015) Apparent diffusion coefficient for discriminating metastatic lymph nodes in patients with squamous cell carcinoma of the head and neck. Diagn Interv Radiol 21:397–402
Dai YL, King AD (2018) State of the art MRI in head and neck cancer. Clin Radiol 73:45–59
Marzi S, Piludu F, Vidiri A (2013) Assessment of diffusion parameters by intravoxel incoherent motion MRI in head and neck squamous cell carcinoma. NMR Biomed 26:1806–1814
Toledano-Massiah S, Luciani A, Itti E, Zerbib P, Vignaud A, Belhadj K, Baranes L, Haioun C, Lin C, Rahmouni A (2015) Whole-body diffusion-weighted imaging in Hodgkin lymphoma and diffuse large B-cell lymphoma. Radiographics 35:747–764
Acknowledgements
We thank Michele Farella, Elisa Tommasini, Pierfrancesco Rinaldi for their continued technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Vidiri, A., Minosse, S., Piludu, F. et al. Cervical lymphadenopathy: can the histogram analysis of apparent diffusion coefficient help to differentiate between lymphoma and squamous cell carcinoma in patients with unknown clinical primary tumor?. Radiol med 124, 19–26 (2019). https://doi.org/10.1007/s11547-018-0940-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0940-1