Abstract
Purpose
The authors analysed the role of diffusion-weighted imaging (DWI) as an additional tool in magnetic resonance (MR) evaluation of prostate cancer.
Materials and methods
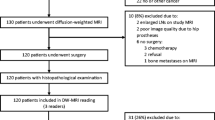
Forty-one patients with suspected prostate cancer underwent MR imaging (1.5 Tesla). A DWI sequence was added to the standard morphological protocol, with a maximum b value of 1,000 s/mm2. Diffusion maps were obtained, and the apparent diffusion coefficient (ADC) was calculated by drawing a region of interest (ROI) over healthy tissue and areas suspicious for malignancy. Histology was considered the gold standard.
Results
The areas correctly classified by MR imaging (42/51) had a low signal intensity on T2-weighted imaging and low ADC value (0.99±0.15 mm2/s; p<0.01) compared with the healthy peripheral zone (PZ) (1.73±0.27 mm2/s; p<0.01). Nine areas classified as suspicious for malignancy on T2-weighted sequences showed high ADC (1.44±0.06 mm2/s; p<0.01) and were confirmed to be disease free by subsequent histological examination. The accuracy of morphofunctional MR imaging was 81.6% compared with 73.7% of the morphological analysis alone.
Conclusions
The addition of DWI to the standard protocol increases the overall diagnostic performance of MR imaging in detecting prostatic cancer. Thus, DWI can help the clinician determine the most appropriate management strategy for the patient.
Riassunto
Obiettivo
Scopo del presente lavoro è stato valutare il ruolo delle sequenze pesate in diffusione (DWI) come strumento addizionale nello studio del carcinoma prostatico in risonanza magnetica (RM).
Materiali e metodi
Quarantuno pazienti con sospetto di neoplasia prostatica sono stati sottoposti a RM (1,5 T). Al protocollo morfologico è stata aggiunta la sequenza single shot (SSh)-DWI sul piano assiale con valore massimo di b pari a 1000 s/mm2. Sono state ricostruite le mappe di diffusione ed il coefficiente di diffusione apparente (ADC) è stato calcolato posizionando le regioni di interesse (ROI) su tessuto sospetto e tessuto sano. L’esame istologico finale è stato considerato il gold standard.
Risultati
Le aree prostatiche correttamente identificate alla valutazione RM come sospette (42 su 51) hanno mostrato basso segnale nelle sequenze T2 pesate ed il corrispondente ADC è risultato statisticamente più basso (0,99±0,15 mm2/s; p<0,01) rispetto al tessuto nella regione periferica sana (1,73±0,27 mm2/s; p<0,01). Nove aree sospette alle sequenze T2 hanno mostrato ADC non tipico per localizzazione cancerose (1,44±0,06 mm2/s; p<0,01): l’esame istologico ha confermato i risultati della DWI. L’accuratezza globale dell’esame RM anatomo-funzionale è stata di 81,6%, più alta rispetto alla sola valutazione morfologica (73,7%).
Conclusioni
Includere la sequenza DWI nel protocollo RM standard migliora la performance diagnostica globale e può aiutare il clinico nel corretto management del paziente.
Similar content being viewed by others
References/Bibliografia
Vilanova JC, Comet J, Garcia-Figueiras R et al (2010) Usefulness of magnetic resonance imaging in prostate cancer. RadiologÌa 52:513–524
Choi YJ, Kim JK, Namkug K et al (2007) Functional MR imaging of prostate cancer. Radiographics 27:63–77
Panebianco V, Sciarra A, Ciccariello M et al (2010) Role of magnetic resonance spectroscopic imaging ([1H]MRSI) and dynamic contrast-enhanced MRI (DCEMRI) in identifying prostate cancer foci in patients with negative biopsy and high levels of prostate-specific antigen (PSA). Radiol Med 115:1314–1329
Renken NS, Krestin GP (2005) Magnetic resonance imaging of the kidney. Semin Ultrasound CT MR 26:153–161
Kilickesmez O, Yirik G, Bayramoglu S et al (2008) Non-breath-hold high b-value diffusion-weighted MRI with parallel imaging technique: apparent diffusion coefficient determination in normal abdominal organs. Diagn Interv Radiol 14:83–87
Kozlowski P, Chang SD, Goldenberg SL (2008) Diffusion-weighted MRI in prostate cancer — comparison between single-shot fast spin echo and echo planar imaging sequences. Magn Reson Imaging 26:72–76
Khoury S. Chatelain C (1992) A comparative classification of urological tumors. FIIS, Paris
Portalez D, Rollin G, Leandri P et al (2010) Prospective comparison of T2w-MRI and dynamic-contrast-enhanced MRI, 3D-MR spectroscopic imaging or diffusion-weighted MRI in repeat TRUS-guided biopsies. Eur Radiol 20:2781–2790
Issa B (2002) In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J Magn Reason Imaging 16:196–200
Kiliçkesmez O, Cimilli T, Inci E et al (2009) Diffusion-weighted MRI of urinary bladder and prostate cancers. Diagn Interv Radiol J 15:104–110
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Le Bihan D, Breton E, Lallemand D et al (1988) Separation of diffusion and perfusion in intravoxel inchoherent motion MR imaging. Radiology 168:497–505
Kitajima K Kaji Y, Kuroda K et al (2008) High b-value diffusion-weighted imaging in normal and malignant peripheral zone tissue of the prostate: effect of signal-to-noise ratio. Magn Reson Med Sci 7:93–99
Bammer R, Auer M, keeling SL et al (2002) Diffusion tensor imaging using single-shot SENSE-EPI. Magn Reson Med 48:128–136
Tamada T, Sone T, Toshimitsu S et al (2008) Age-related and zonal anatomical changes of apparent diffusion coefficient values in normal human prostatic tissues. J Magn Reson Imaging 27:552–556
Haider MA, Van der Kwast TH, Tanguay J et al (2007) T2-weghted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol 189:323–328
Miao H, Fukatsu H, Ishigaki T (2007) Prostate cancer detection with 3-T MRI: comparison of diffusion-weighted and T2-weighted imaging. Eur J Radiol 61:297–302
Reinsberg SA, Payne GS, Riches SF et al (2007) Combined use of diffusion.weighted MRI and 1H MR Spectroscopy to increase accuracy in prostate cancer detection. AJR Am J Roentgenol 188:91–98
Kim JK, Jang YJ, Cho G (2009) Multidisciplinary functional MR imaging for prostate cancer. Korean J Radiol 10:535–551
Petralia G, Thoeny HC (2010) DW-MRI of the urogenital tract: applications in oncology. Cancer Imaging 10(Spec no A):S112–S123
Afaq A, Koh DM, Padhani A et al (2011) Clinical utility of diffusion-weighted magnetic resonance imaging in prostate cancer. BJU Int 108:1716–1722
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rinaldi, D., Fiocchi, F., Ligabue, G. et al. Role of diffusion-weighted magnetic resonance imaging in prostate cancer evaluation. Radiol med 117, 1429–1440 (2012). https://doi.org/10.1007/s11547-012-0832-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-012-0832-8