Abstract
Purpose
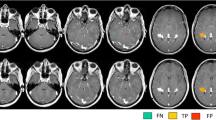
This study was undertaken to assess the value of a chemical (spectral) fat-saturation (fat-sat) pulse added to a T1-weighted spin-echo sequence after intravenous administration of paramagnetic contrast agent in detecting enhancing lesions in multiple sclerosis.
Materials and methods
Twenty patients with relapsing-remitting multiple sclerosis underwent a brain 1.0-Tesla magnetic resonance (MR) scan with T1-weighted spin-echo sequences (24 contiguous para-axial slices with a thickness of 5 mm, pixel size 0.96 mm2, number of excitations 2, flip angle 90°) 5 min after intravenous injection of 0.1 mmol/kg of gadodiamide with and without fat-sat, acquired with randomised order of priority. Two readers counted by consensus the number of enhancing lesions and assigned a conspicuity score (low conspicuity=1; high conspicuity=2) to each enhancing lesion during a randomised reading without any visual comparison between the two corresponding images (with and without fat-sat) of the same patient. McNemar and Wilcoxon matched-pair signed-rank tests were used.
Results
Seventy-two enhancing lesions without fat-sat and 94 with fat-sat were detected; 22 lesions were visible only with fatsat, whereas no lesion was detected only without fat-sat (p<0.0001). The conspicuity score was 1.17±0.38 (mean±standard deviation) and 1.57±0.44, respectively (p<0.0001).
Conclusions
A fat-sat pulse added to a T1-weighted spin-echo sequence increases significantly the number and conspicuity of contrast-enhancing lesions in patients with relapsing-remitting multiple sclerosis.
Riassunto
Obiettivo
Confrontare sequenze spin-echo T1-pesate con e senza saturazione spettrale del grasso dopo somministrazione endovenosa di mezzo di contrasto paramagnetico per il riconoscimento di lesioni encefaliche con contrast-enhancement in pazienti affetti da sclerosi multipla.
Materiali e metodi
Venti pazienti affetti da sclerosi multipla intermittente-remittente sono stati sottoposti a RM encefalica a 1 Tesla con sequenze spin-echo T1-pesate (24 strati para-assiali contigui, spessore 5 mm, pixel 0,96 mm2, 2 eccitazioni, angolo di nutazione 90o) cinque minuti dopo iniezione endovenosa di 0,1 mmol/kg di gadodiamide, con e senza saturazione del grasso, acquisite in ordine temporale randomizzato. Due lettori in consenso hanno contato per entrambe le sequenze, con lettura randomizzata e senza confronto visuale tra le due immagini corrispondenti (con e senza saturazione del grasso) dello stesso paziente, il numero di lesioni focali con contrast-enhancement, assegnando a ciascuna un punteggio di cospicuità (bassa cospicuità=1; alta cospicuità=2). Per l’analisi statistica sono stati utilizzati i test di McNemar e Wilcoxon per ranghi per dati appaiati.
Risultati
Mentre la sequenza senza saturazione del grasso ha consentito il riconscimento di 72 lesioni, quella con saturazione del grasso ha consentito il riconoscimento di 94 lesioni: 22 lesioni erano quindi riconoscibili alla sola sequenza con saturazione del grasso (p<0,0001). Il punteggio di cospicuità è risultato pari a 1,17±0,38 (media±deviazione standard) e 1,57±0,44, rispettivamente (p<0,0001).
Conclusioni
L’integrazione di un preimpulso di saturazione del grasso in una sequenza spin-echo T1-pesata consente di ottenere un significativo incremento del numero e della cospicuità delle lesioni encefaliche con contrast-enhancement in pazienti affetti da sclerosi multipla intermittente-remittente.
Similar content being viewed by others
References/Bibliografia
Fazekas F, Barkhof F, Filippi M et al (1999) The contribution of magnetic resonance imaging to the diagnosis of multiple sclerosis. Neurology 53:448–456
Katz D, Taubenberger JK, Cannella B et al (1993) Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol 34:661–669
Barkhof F, Filippi M, Miller DH et al (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120:2059–2069
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 50:121–127
Molyneux PD, Miller DH (1999) Magnetic resonance imaging techniques to monitor phase III treatment trials. In: Filippi M, Grossman RI, Comi G (eds) Magnetic resonance techniques in clinical trials in multiple sclerosis. Springer, Milano, pp 49–73
Losseff N, Kingsley DPE, McDonald WI et al (1996) Clinical and magnetic resonance predictors of disability in primary and secondary progressive multiple sclerosis. Mult Scler 1:218–222
Koudriavtseva T, Thompson AJ, Fiorelli M et al (1997) Gadolinium enhanced MRI disease activity in relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 62:285–287
Filippi M (2000) Enhanced magnetic resonance imaging in multiple sclerosis. Mult Scler 6:320–326
Sardanelli F, Losacco C, Iozzelli A et al (2002) Evaluation of Gd-enhancement in brain MR of multiple sclerosis: image subtraction with and without magnetization transfer. Eur Radiol 12:2077–2082
Sardanelli F, Iozzelli A, Losacco C et al (2003) Three subsequent single doses of Gd-chelate in brain MR of multiple sclerosis. AJNR Am J Neuroradiol 24:658–662
Delfaut EM, Beltran J, Johnson G et al (1999) Fat suppression in MR imaging: techniques and pitfalls. Radiographics 19:373–382
Fujii Y, Konishi Y, Kuriyama M et al (1993) Lipoma on surface of centroparietal lobes. Pediatr Neurol 9:144–166
Dahlen RT, Johnson CE, Harnsberger HR et al (2002) CT and MR imaging characteristics of intravestibular lipoma. AJNR Am J Neuroradiol 23:1413–1417
Sener RN (1995) Isolated choroid plexus lipomas. Comput Med Imaging Graph 19:423–426
Mirowitz SA, Apicella P, Reinus WR, Hammerman AM (1994) MR imaging of bone marrow lesions: relative conspicuousness on T1-weighted, fatsuppressed T2-weighted, and STIR images. AJR Am J Roentgenol 162:215–221
Seitz J, Held P, Strotzer M et al (2002) MR imaging of cranial nerve lesions using six different high-resolution T1- and T2(*)-weighted 3D and 2D sequences. Acta Radiol 43:349–353
Guy J, Mao J, Bidgood WD Jr, Mancuso A, Quisling RG (1992) Enhancement and demyelination of the intraorbital optic nerve. Fat suppression magnetic resonance imaging. Ophthalmology 99:713–719
Sklar EM, Schatz NJ, Glaser JS et al (1996) MR of vasculitis-induced optic neuropathy. AJNR Am J Neuroradiol 17:121–128
Jackson A, Sheppard S, Laitt RD et al (1998) Optic neuritis: MR imaging with combined fat- and water-suppression techniques. Radiology 206:57–63
Okamoto K, Ito J, Ogawa R et al (1999) Bilateral optic neuritis in a child diagnosed with Gd-enhanced MR imaging using fat-suppression technique. Eur Radiol 9:731–733
Barkhof F (1996) Role of MR imaging in the diagnosis of MS. Ad MRI Contrast 4:31–38
Namer IJ, Yu O, Mauss Y, Dumitresco BE, Chambron J (1993) An evaluation of the significance of areas of intense signal in the MR brain images of patients with multiple sclerosis. Magn Reson Imaging 11:311–317
Hashemi RH, Bradley WG, Chen DY et al (1995) Suspected multiple sclerosis: MR imaging with a thin-section fast Flair pulse sequence. Radiology 196:505–510
Filippi M, Yousry T, Baratti C et al (1996) Quantitative assessment of MRI lesion load in multiple sclerosis. A comparison of conventional spin-echo with fast fluid-attenuated inversion recovery. Brain 119:1349–1355
Truyen L, van Waesberghe JH, van Walderveen MA et al (1996) Accumulation of hypointense lesion (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology 47:1469–1476
van Waesberghe JH, Kamphorst W, De Groot CJ et al (1999) Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 46:747–754
Van Waesberghe JH (1996) Magnetization transfer ratio and contrast in multiple sclerosis. Ad MRI contrast 4:54–60
Renzetti P, Parodi RC, Losacco C et al (1999) Brain magnetic resonance with magnetization transfer in multiple sclerosis. Lesion hyperintensity before and after intravenous gadolinium administration. Radiol Med 98:138–143
Bastianello S, Gasperini C, Paolillo A et al (1998) Sensitivity of enhanced MR in multiple sclerosis: effects of contrast dose and magnetization transfer contrast. AJNR Am J Neuroradiol 19:1863–1867
Filippi M, Rovaris M, Capra R et al (1998) A multi-centre longitudinal study comparing the sensitivity of monthly MRI after standard and triple dose gadolinium-DTPA for monitoring disease activity in multiple sclerosis. Implications for phase II clinical trials. Brain 121:2011–2020
Rovaris M, Codella M, Moiola L et al (2002) Effect of glatiramer acetate on MS lesions enhancing at different gadolinium doses. Neurology 59:1429–1432
Mancardi GL, Saccardi R, Filippi M et al (2001) Cell transplantation for multiple sclerosis. Autologous hematopoietic stem cell transplantation suppresses Gd-enhanced MRI activity in MS. Neurology 57:62–68
Rovaris M, Filippi M (2000) Contrast enhancement and the acute lesion in multiple sclerosis. Neuroimaging Clin N Am 10:705–716
Mao J, Yan H, Brey WW (1993) Fat tissue and fat suppression. Magn Reson Imaging 11:385–393
Jackson EF, Ginsberg LE, Schomer DF, Leeds NE (1997) A review of MRI pulse sequences and techniques in neuroimaging. Surg Neurol 47:185–199
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sardanelli, F., Schiavoni, S., Iozzelli, A. et al. The value of chemical fat-saturation pulse added to T1-weighted spin-echo sequence in evaluating gadolinium-enhancing brain lesions in multiple sclerosis. Radiol med 112, 1244–1251 (2007). https://doi.org/10.1007/s11547-007-0220-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-007-0220-y