Abstract
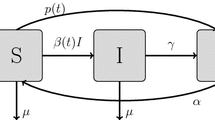
We use the reinfection SIRI epidemiological model to analyze the impact of education programs and vaccine scares on individuals decisions to vaccinate or not. The presence of the reinfection provokes the novelty of the existence of three Nash equilibria for the same level of the morbidity relative risk instead of a single Nash equilibrium as occurs in the SIR model studied by Bauch and Earn (PNAS 101:13391–13394, 2004). The existence of three Nash equilibria, with two of them being evolutionary stable, introduces two scenarios with relevant and opposite features for the same level of the morbidity relative risk: the low-vaccination scenario corresponding to the evolutionary stable vaccination strategy, where individuals will vaccinate with a low probability; and the high-vaccination scenario corresponding to the evolutionary stable vaccination strategy, where individuals will vaccinate with a high probability. We introduce the evolutionary vaccination dynamics for the SIRI model and we prove that it is bistable. The bistability of the evolutionary dynamics indicates that the damage provoked by false scares on the vaccination perceived morbidity risks can be much higher and much more persistent than in the SIR model. Furthermore, the vaccination education programs to be efficient they need to implement a mechanism to suddenly increase the vaccination coverage level.







Similar content being viewed by others
References
Aguiar M, Kooi B, Stollenwerk N (2008) Epidemiology of dengue fever: a model with temporary cross-immunity and possible secondary infection shows bifurcations and chaotic behaviour in wide parameter regions. Math Model Nat Phenom 3:48–70
Alexander ME, Bowman C, Moghadas SM, Summers R, Gumel AB, Sahai BM (2004) A vaccination model for transmission dynamics of influenza. SIAM J Appl Dyn Syst 3:503–524
Andreasen V, Lin J, Levin SA (1997) The dynamics of cocirculating influenza strains conferring partial cross-immunity. J Math Biol 35:825–842
Basu S, Chapman GB, Galvani AP (2008) Integrating epidemiology, psychology, and economics to achieve HPV vaccination targets. PNAS 105:19018–19023
Bauch CT, Earn DJD (2004) Vaccination and the theory of games. PNAS 101:13391–13394
Bauch CT (2005) Imitation dynamics predict vaccinating behaviour. Proc R Soc Lond B 272:1669–1675
Bauch CT, Bhattacharyya S (2012) Evolutionary game theory and social learning can determine how vaccine scares unfold. PLoS Comput Biol 8(4):e1002452
Buonomo B, d’Onofrio A, Lacitignola D (2008) Global stability of an SIR epidemic model with information dependent vaccination. Math Biosci 216:9–16
Chamchod F, Britton NF (2012) On the dynamics of a two-strain influenza model with isolation. Math Model Nat Phenom 7:49–61
Chen FH (2006) A susceptible-infected epidemic model with voluntary vaccinations. J Math Biol 53:253–272
Cojocaru MG, Bauch CT, Johnston MD (2007) Dynamics of vaccination strategies via projected dynamical systems. Bull Math Biol 69(5):1453–1476
Cojocaru M, Bauch CT (2009) Vaccination strategy dynamics of population groups with distinct perceived probability of infection. J Inequal Pure Appl Math 10(1):1–16
Davies J, Grilli E, Smith A (1983) Influenza A: infection and reinfection. J Hyg (Cambridge) 92:125–127
Ferguson NM, Galvani AP, Bush RM (2003) Ecological and immunological determinants of influenza evolution. Nature 422:428–433
Fine PEM, Clarkson JA (1986) Individual versus public priorities in the determination of optimal vaccination policies. Am J Epidemiol 124(6):1012–1020
Galvani AP, Reluga TC, Chapman GB (2007) Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. Proc Natl Acad Sci 104:5692–5697
Gomes MG, White LJ, Medley GF (2004) Infection, reinfection, and vaccination under suboptimal immune protection: epidemiological perspectives. J Theor Biol 228:539–549
Gomes MG, Franco AO, Gomes MC, Medley GF (2004) The reinfection threshold promotes variability in tuberculosis epidemiology and vaccine efficacy. Proc R Soc Lond B 271:617–623
Gomes MG, White LJ, Medley GF (2005) The reinfection threshold. J Theor Biol 236:111–113
Gupta S, Maiden MCJ (2001) Exploring the evolution of diversity in pathogen populations. Trends Microbiol 9:181–185
Hay AJ, Gregory V, Douglas AR, Lin YP (2001) The evolution of human influenza viruses. Philos Trans R Soc London B 356:1861–1870
Heesterbeek JAP (2002) A brief history of R0 and a recipe for its calculation. Acta Biotheor 50:189–204
Hofbauer J, Sigmund K (1998) Evolutionary games and population dynamics. Cambridge University Press, Cambridge
Kermack WO, McKendrick AG (1927) A contribution to the mathematical theory of epidemics. Proc Roy Soc Lond 115:700–721
Lipsitch M (1997) Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci USA 94:6571–6576
Liu J, Kochin BF, Tekle YI, Galvani AP (2012) Epidemiological game-theory dynamics of chickenpox vaccination in the USA and Israel. J R Soc Interface 9:68–76
Maynard-Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
Moghadas SM (2004) Modelling the effect of imperfect vaccines on disease epidemiology. Discrete Contin Dyn Syst Ser B 4:999–1012
Moreira HN, Wang Y (1997) Global stability in an S \(\rightarrow \) I \(\rightarrow \) R \(\rightarrow \) I model. SIAM Rev 39:496–502
Nowak M (2006) Evolutionary dynamics: exploring the equations of life. Belknap Press, Cambridge
Nuño N, Feng Z, Martcheva M, Castillo-Chavez C (2005) Dynamics of two-strain influenza with isolation and partial cross-immunity. SIAM J Appl Math 65:964–982
Palese P, Young JF (1982) Variation of influenza A, B, and C viruses. Science 215:1486–1474
Reluga TC, Bauch CT, Galvani AP (2006) Evolving public perceptions and stability in vaccine uptake. Math Biosci 204(2):185–198
Reluga TC (2009) An SIS epidemiology game with two subpopulations. J Biol Dyn 3:515–531
Reluga TC, Galvani AP (2011) A general approach for population games with application to vaccination. Math Biosci 230(2):67–78
Shim E, Chapman GB, Townsend JP, Galvani AP (2012) The influence of altruism on influenza vaccination decisions. J R Soc Interface 9:2234–2243
Song L-P, Jin Z, Sun G-Q (2011) Reinfection induced disease in a spatial SIRI model. J Biol Phys 37:133–140
Sonoguchi T, Sakoh M, Kunita N, Satsuta K, Noriki H, Fukumi H (1986) Reinfection with influenza A (H2N2, H3N2, and H1N1) viruses in soldiers and students in Japan. J Infect Dis 153:33–40
Stollenwerk N, Jansen V (2010) Population biology and criticality: from critical birth-death processes to self-organized criticality in mutation pathogen systems. World Scientific, Sigapore
Stollenwerk N, Martins J, Pinto A (2007) The phase transition lines in pair approximation for the basic reinfection model SIRI. Phys Lett A 371:379–388
Stollenwerk N, van Noort S, Martins J, Aguiar M, Hilker F, Pinto A, Gomes MG (2010) A spatially stochastic epidemic model with partial immunization shows in mean field approximation the reinfection threshold. J Biol Dyn 4:634–649
Tudor D (1990) A deterministic model for herpes infections in human and animal populations. SIAM Rev 32:136–139
van den Driessche P, Wang L, Zou X (2007) Modeling diseases with latency and relapse. Math Biosci Eng 4:205–219
van den Driessche P, Zou X (2007) Modeling relapse in infectious diseases. Math Biosci 207:89–103
Acknowledgements
The authors thank the referees for their comments and suggestions. The authors also thank the financial support of LIAAD-INESC TEC and FCT Fundação para a Ciência e a Tecnologia (Portuguese Foundation for Science and Technology) within project UID/EEA/50014/2013 and ERDF (European Regional Development Fund) through the COMPETE Program (operational program for competitiveness) and by National Funds through the FCT within Project “Dynamics, optimization and modelling”, with reference PTDC/MAT-NAN/6890/2014 and Project “NanoSTIMA: Macro-to-Nano Human Sensing: Towards Integrated Multimodal Health Monitoring and Analytics/NORTE-01-0145-FEDER-000016” financed by the North Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, and through the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, J., Pinto, A. Bistability of Evolutionary Stable Vaccination Strategies in the Reinfection SIRI Model. Bull Math Biol 79, 853–883 (2017). https://doi.org/10.1007/s11538-017-0257-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0257-6