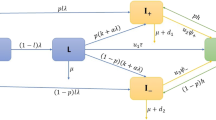
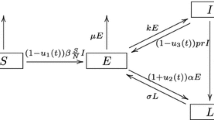
Abstract
We propose and analyze an optimal control problem where the control system is a mathematical model for tuberculosis that considers reinfection. The control functions represent the fraction of early latent and persistent latent individuals that are treated. Our aim was to study how these control measures should be implemented, for a certain time period, in order to reduce the number of active infected individuals, while minimizing the interventions implementation costs. The optimal intervention is compared along different epidemiological scenarios, by varying the transmission coefficient. The impact of variation of the risk of reinfection, as a result of acquired immunity to a previous infection for treated individuals on the optimal controls and associated solutions, is analyzed. A cost-effectiveness analysis is done, to compare the application of each one of the control measures, separately or in combination.





Similar content being viewed by others
References
AMPL (A Mathematical Programming Language). http://www.ampl.com
Blower S, Small P, Hopewell P (1996) Control strategies for tuberculosis epidemics: new models for old problems. Science 273:497–500
Castillo-Chavez C, Feng Z (1997) To treat or not to treat: the case of tuberculosis. J Math Biol 35:629–656
Castillo-Chavez C, Feng Z (1998) Mathematical models for the disease dynamics of tuberculosis. In: Horn MA, Simonett G, Webb GF (eds) Advances in mathematical population dynamics—molecules, cells and man. Vanderbilt University Press, Nashville, pp 117–128
Cesari L (1983) Optimization—theory and applications. Applications of mathematics. Springer, New York
Cohen T, Murray M (2004) Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med 10:1117–1121
Cohen T, Colijn C, Finklea B, Murray M (2007) Exogenous re-infection in tuberculosis: local effects in a network model of transmission. J R Soc Interface 4:523–531
Dye C, Garnett GP, Sleeman K, Williams BG (1998) Prospects for worldwide tuberculosis control under the who dots strategy. Directly observed short-course therapy. Lancet 352:1886–1891
Fleming WH, Rishel RW (1975) Deterministic and stochastic optimal control. Springer, New York
Gomes M, Franco A, Gomes M, Medley G (2004) The reinfection threshold promotes variability in tuberculosis epidemiology and vaccine efficacy. Proc R Soc B 271:617–623
Gomes MGM, Rodrigues P, Hilker FM, Mantilla-Beniers NB, Muehlen M, Paulo AC, Medley GF (2007) Implications of partial immunity on the prospects for tuberculosis control by post-exposure interventions. J Theor Biol 248:608–617
IPOPT (Interior Point OPTimizer). https://projects.coin-or.org/Ipopt
Jung E, Lenhart S, Feng Z (2002) Optimal control of treatments in a two-strain tuberculosis model. Discrete Continuous Dyn Syst Ser B 2:473–482
Kar TK, Jana S (2013) A theoretical study on mathematical modelling of an infectious disease with application of optimal control. BioSystems 111:37–50
Moualeu DP, Weiser M, Ehrig R, Deuflhard P (2015) Optimal control for a tuberculosis model with undetected cases in Cameroon. Commun Nonlinear Sci Numer Simul 20:986–1003
Okosun KO, Rachid O, Marcus N (2013) Optimal control strategies and cost-effectiveness analysis of a malaria model. BioSystems 111:83–101
Pontryagin L, Boltyanskii V, Gramkrelidze R, Mischenko E (1962) The mathematical theory of optimal processes. Wiley, New York
PROPT—Matlab Optimal Control Software (DAE, ODE). https://tomdyn.com
Rie AV, Zhemkov V, Granskaya J, Steklova L, Shpakovskaya L, Wendelboe A, Kozlov A, Ryder R, Salfinger M (2005) TB and HIV in St Petersburg, Russia: a looming catastrophe? Int J Tuberc Lung Dis 9:740–745
Rodrigues P, Rebelo C, Gomes MGM (2007) Drug resistance in tuberculosis: a reinfection model. Theor Popul Biol 71:196–212
Rodrigues HS, Monteiro MTT, Torres DFM (2014) Optimal control and numerical software: an overview. In: Miranda F (ed) Systems theory: perspectives, applications and developments. Nova Science, New York, pp 93–110
Rodrigues P, Silva CJ, Torres DFM (2013) Optimal control strategies for reducing the number of active infected individuals with tuberculosis. In: Proceedings of the SIAM conference on control and its applications (CT13), San Diego, CA, USA, July 8–10, pp 44–50
Silva CJ, Torres DFM (2012) Optimal control strategies for tuberculosis treatment: a case study in Angola. Numer Algebra Control Optim 2(3):601–617
Silva CJ, Torres DFM (2013) Optimal control for a tuberculosis model with reinfection and post-exposure interventions. Math Biosci 244(2):154–164
Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, Enarson DA, Behr MA, van Helden PD (2005) Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 171:1430–1435
Vynnycky E, Fine PE (1997) The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect 119:183–201
Warren RM, Victor TC, Streicher EM, Richardson M, Beyers N, Pittius NCG, Helden PD (2004) Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med 169:610–614
WHO (2013) Global tuberculosis report 2013. World Health Organization, Geneva. http://www.who.int/tb/publications/global_report/en
Acknowledgments
This work was partially supported by the Portuguese Foundation for Science and Technology (FCT) through the Centro de Matemática e Aplicações, Project PEst-OE/MAT/UI0297/2014 (Rodrigues); Center for Research and Development in Mathematics and Applications (CIDMA), Project PEst-OE/MAT/UI4106/2014 (Silva and Torres); postdoc fellowship SFRH/BPD/72061/2010 (Silva); Project PTDC/EEI-AUT/1450/2012, co-financed by FEDER under POFC-QREN with COMPETE reference FCOMP-01-0124-FEDER-028894 (Torres). The authors are grateful to Gabriela Gomes for stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Proof of Theorem 3.1
The Hamiltonian \(H\) associated with the problem (1)–(3) is given by
where \(\lambda (t) = \left( \lambda _1(t), \lambda _2(t), \lambda _3(t), \lambda _4(t), \lambda _5(t)\right) \) is the adjoint vector. According to the Pontryagin maximum principle (Pontryagin et al. 1962), if \((u_1^*(\cdot ), u_2^*(\cdot )) \in \Omega \) is optimal for problem (1)–(3) with the initial conditions given in Table 2 and fixed final time \(t_f\), then there exists a non-trivial absolutely continuous mapping \(\lambda : [0, t_f] \rightarrow \mathbb {R}^5\), \(\lambda (t) = \left( \lambda _1(t), \lambda _2(t), \lambda _3(t), \lambda _4(t), \lambda _5(t)\right) \), such that
and
The minimality condition
holds almost everywhere on \([0, t_f]\). Moreover, the transversality conditions
hold.
Lemma
For problem (1)–(3) with fixed initial conditions \(S(0)\), \(L_1(0)\), \(I(0)\), \(L_2(0)\) and \(R(0)\) and fixed final time \(t_f\), there exists adjoint functions \(\lambda _1^*(\cdot )\), \(\lambda _2^*(\cdot )\), \(\lambda _3^*(\cdot )\), \(\lambda _4^*(\cdot )\) and \(\lambda _5^*(\cdot )\) such that
with transversality conditions
Furthermore,
Proof
System (11) is derived from the Pontryagin maximum principle (see (9), Pontryagin et al. 1962) and the optimal controls (12) come from the minimality condition (10). For small final time \(t_f\), the optimal control pair given by (12) is unique due to the boundedness of the state and adjoint functions and the Lipschitz property of systems (1) and (11) (see Jung et al. 2002 and references cited therein). \(\square \)
Proof of Theorem 3.1
Existence of an optimal solution \(\left( S^*, L_1^*, I^*, L_2^*, R^*\right) \) associated with an optimal control pair \(\left( u_1^*, u_2^*\right) \) comes from the convexity of the integrand of the cost functional \(\mathcal {J}\) with respect to the controls \((u_1, u_2)\) and the Lipschitz property of the state system with respect to state variables \(\left( S, L_1, I, L_2, R\right) \) (see, e.g., Cesari 1983; Fleming and Rishel 1975). For small final time \(t_f\), the optimal control pair is given by (12) that is unique by the Lemma above. Because the problem (1)–(3) is autonomous, uniqueness is valid for any time \(t_f\) and not only for small time \(t_f\). \(\square \)
Appendix 2: Sensitivity Analysis to the Duration of Intervention \(t_f\)
We fix \(\beta =100\) and \(\sigma _R=\sigma \) and the remaining parameters according to Table 1 and vary \(t_f\). Results for the proportion of infectious individuals are shown in the Fig. 6. The general behavior do not change significantly with \(t_f\). The proportion of infected individuals slightly increases toward the end of the intervention for \(t_f>7\). This tendency is more pronounced for higher \(t_f\).
Proportion of infectious individuals for the optimal solution \(I(t)\) with \(t_f \in \{5, 7, 10, 12, 15, 17, 20, 22, 25 \}\). Parameters according to Table 1, \(\beta =100\) and \(\sigma _R=\sigma \)
Appendix 3: Sensitivity Analysis to the Weight Constants on the Objective Functional \(\mathcal {J}\)
Figure 7 shows the results for different combination of the weight constants on the objective functional \(\mathcal {J}\). We fix \(\beta =100\) and \(\sigma _R=\sigma \) and the remaining parameters according to Table 1 and vary \(W_0\), \(W_1\) and \(W_2\). Efficacy decreases when the costs \(W_1\) and \(W_2\) increase, corresponding to an earlier relaxation of the intensity of treatment \((u_1(t), u_2(t))\) in the optimal solution due to cost restrictions. The change in efficacy is more pronounced for the cases where the weight associated with infectious individuals \(W_0\) change in comparison with the weights associated with the controls \(W_1=W_2\) (Fig. 7a, b). Results are less sensitive to the variation between the weight controls \(W_1\) and \(W_2\) (Fig. 7c, d).
Sensitivity analysis to the weight constants on the objective functional \(\mathcal {J}\). a \(W_0=50\) and \(W_1=W_2=5, 25, 50, 100, 200, 500\). b \(W_1=W_2=50\) and \(W_0=5, 25, 50, 100, 200, 500\). c \(W_0=W_2=50\) and \(W_1=5, 25, 50, 100, 200, 500\). d \(W_0=W_1=50\) and \(W_2=5, 25, 50, 100, 200, 500\)
Rights and permissions
About this article
Cite this article
Rodrigues, P., Silva, C.J. & Torres, D.F.M. Cost-Effectiveness Analysis of Optimal Control Measures for Tuberculosis. Bull Math Biol 76, 2627–2645 (2014). https://doi.org/10.1007/s11538-014-0028-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0028-6