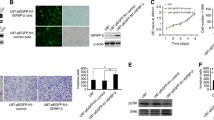
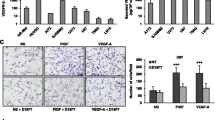
Abstract
Background
High-grade meningiomas (HGMs; World Health Organization [WHO] classification grade II and III) have high relapse rates and poor clinical outcomes despite surgery and radiation treatments. No effective medical therapy currently exists for HGMs, and developing novel therapeutic strategies depends on the identification of molecular drivers. In cancer, β1 integrin enhances malignant characteristics, including proliferation, invasion, and drug resistance.
Objective
We conducted this study to investigate whether β1 integrin could be a therapeutic target in HGMs.
Patients and Methods
Expression of β1 integrin was examined in gene array datasets, with proteomics of clinical meningioma specimens, and in patient-derived HGM xenografts. Anti-tumor activity of OS2966, a first-in-class humanized antagonizing monoclonal antibody against β1 integrin, was tested in vitro and in vivo using an orthotopic mouse model of patient-derived malignant meningioma.
Results
β1 integrin was expressed in meningiomas of all WHO grades and two xenografts tested. In vitro, OS2966 suppressed the viability of NF2-deficient MN3 sphere cells and NF2-wild-type IOMM-Lee malignant meningioma cells only when plated on laminin-coated plastic. While OS2966 decreased phosphorylation of ERK1/2 in both MN3 cells and laminin-grown IOMM-Lee cells, OS2966 only affected the phosphorylation of FAK (Tyr397) in MN3, and of Akt (Ser473) in IOMM-Lee cells, respectively, indicating differential pathway inhibition. Systemic administration of OS2966 in mice bearing orthotopic MN3 HGMs inhibited HGM cell proliferation and significantly extended overall survival of the treated mice.
Conclusions
β1 Integrin may be a therapeutic target in HGMs, and further preclinical and clinical development of OS2966 for HGM therapy is warranted.





Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4th ed. Lyon: IARC; 2016 (revised).
Preusser M, Brastianos PK, Mawrin C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. 2018;14(2):106–15.
Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20 Suppl 4:iv1–86.
Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 2015;17(8):1166–73.
Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–91.
Buttrick S, Shah AH, Komotar RJ, Ivan ME. Management of atypical and anaplastic meningiomas. Neurosurg Clin N Am. 2016;27(2):239–47.
Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD. Medical therapies for meningiomas. J Neurooncol. 2010;99(3):365–78.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22.
Hamidi H, Pietila M, Ivaska J. The complexity of integrins in cancer and new scopes for therapeutic targeting. Br J Cancer. 2016;115(9):1017–23.
Raab-Westphal S, Marshall JF, Goodman SL. Integrins as therapeutic targets: successes and cancers. Cancers (Basel). 2017;9(9):110.
Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8(8):604–17.
Jahangiri A, Aghi MK, Carbonell WS. Beta1 integrin: critical path to antiangiogenic therapy resistance and beyond. Cancer Res. 2014;74(1):3–7.
Carbonell WS, DeLay M, Jahangiri A, Park CC, Aghi MK. Beta1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 2013;73(10):3145–54.
Beschet I, Brunon J, Scoazec JY, Mosnier JF. Expression of beta1 and beta4 integrins in normal arachnoid membrane and meningiomas. Cancer. 1999;86(12):2649–58.
Figarella-Branger D, Roche PH, Daniel L, Dufour H, Bianco N, Pellissier JF. Cell-adhesion molecules in human meningiomas: correlation with clinical and morphological data. Neuropathol Appl Neurobiol. 1997;23(2):113–22.
Gogineni VR, Nalla AK, Gupta R, Gujrati M, Klopfenstein JD, Mohanam S, et al. alpha3beta1 integrin promotes radiation-induced migration of meningioma cells. Int J Oncol. 2011;38(6):1615–24.
Salehi F, Jalali S, Alkins R, Lee JI, Lwu S, Burrell K, et al. Proteins involved in regulating bone invasion in skull base meningiomas. Acta Neurochir (Wien). 2013;155(3):421–7.
Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25(44):5960–8.
Shapiro IM, Kolev VN, Vidal CM, Kadariya Y, Ring JE, Wright Q, et al. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med. 2014;6(237):237ra68.
Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66(3):1526–35.
Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL, et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Investig. 2014;124(10):4614–28.
Nitta H, Yamashima T, Yamashita J, Kubota T. An ultrastructural and immunohistochemical study of extracellular matrix in meningiomas. Histol Histopathol. 1990;5(3):267–74.
Nigim F, Esaki S, Hood M, Lelic N, James MF, Ramesh V, et al. A new patient-derived orthotopic malignant meningioma model treated with oncolytic herpes simplex virus. Neuro Oncol. 2016;18(9):1278–87.
Lee WH. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery. 1990;27(3):389–95 (discussion 96).
Lee Y, Liu J, Patel S, Cloughesy T, Lai A, Farooqi H, et al. Genomic landscape of meningiomas. Brain Pathol. 2010;20(4):751–62.
Clark VE, Harmanci AS, Bai H, Youngblood MW, Lee TI, Baranoski JF, et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48(10):1253–9.
Harmanci AS, Youngblood MW, Clark VE, Coskun S, Henegariu O, Duran D, et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2017;8:14433.
Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271(42):26329–34.
Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516–23.
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14(3):1680–8.
Schlager C, Korner H, Krueger M, Vidoli S, Haberl M, Mielke D, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530(7590):349–53.
Weller RO, Sharp MM, Christodoulides M, Carare RO, Mollgard K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018;135(3):363–85.
Kalamarides M, Peyre M, Giovannini M. Meningioma mouse models. J Neurooncol. 2010;99(3):325–31.
Mawrin C. Animal models of meningiomas. Chin Clin Oncol. 2017;6(Suppl 1):S6.
Mei Y, Bi WL, Greenwald NF, Agar NY, Beroukhim R, Dunn GP, et al. Genomic profile of human meningioma cell lines. PLoS ONE. 2017;12(5):e0178322.
Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30(5):E6.
Lomas J, Bello MJ, Arjona D, Alonso ME, Martinez-Glez V, Lopez-Marin I, et al. Genetic and epigenetic alteration of the NF2 gene in sporadic meningiomas. Genes Chromosomes Cancer. 2005;42(3):314–9.
Hersey P, Sosman J, O’Day S, Richards J, Bedikian A, Gonzalez R, et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or − dacarbazine in patients with stage IV metastatic melanoma. Cancer. 2010;116(6):1526–34.
Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27(4):574–88.
Lesniak D, Xu Y, Deschenes J, Lai R, Thoms J, Murray D, et al. Beta1-integrin circumvents the antiproliferative effects of trastuzumab in human epidermal growth factor receptor-2-positive breast cancer. Cancer Res. 2009;69(22):8620–8.
Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68(11):4398–405.
Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5(6):662–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Meningioma Mommas (HW and RLM).
Conflict of interest
W. Shawn Carbonell is a co-founder, Director, and CEO of OncoSynergy, Inc. Daniel P. Cahill serves as a consultant for Merck and Lilly. Priscilla K. Brastianos serves as a consultant for Angiochem, Tesaro, and Lilly; has received speaker’s honoraria from Merck and Genentech-Roche; and has received institutional funding from Pfizer and Merck. Fares Nigim, Juri Kiyokawa, Alessandra Gurtner, Yoichiro Kawamura, Lingyang Hua, Ekkehard M. Kasper, Samuel D. Rabkin, Robert L. Martuza, and Hiroaki Wakimoto declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethical approval
All work with animals was conducted with the approval of the IACUC at MGH, and followed all applicable international, national and institutional guidelines for the care and use of animals. The procedures involving human excess materials to establish xenograft models were performed under Institutional Review Board (IRB) approval at MGH in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The IRB protocol exempted informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nigim, F., Kiyokawa, J., Gurtner, A. et al. A Monoclonal Antibody Against β1 Integrin Inhibits Proliferation and Increases Survival in an Orthotopic Model of High-Grade Meningioma. Targ Oncol 14, 479–489 (2019). https://doi.org/10.1007/s11523-019-00654-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00654-4