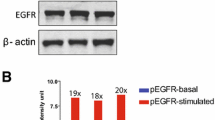
Abstract
Background
Given that aberrant activation of epidermal growth factor receptor family receptors (ErbB) is a common event in oral squamous cell carcinoma, and that high expression of these receptor proteins is often associated with poor prognosis, this rationalizes the approach of targeting ErbB signaling pathways to improve the survival of patients with oral squamous cell carcinoma. However, monotherapy with the ErbB blocker afatinib has shown limited survival benefits.
Objectives
This study was performed to identify mechanisms of afatinib resistance and to explore potential afatinib-based combination treatments with other targeted inhibitors in oral squamous cell carcinoma.
Methods
We determined the anti-proliferative effects of afatinib on a panel of oral squamous cell carcinoma cell lines using a crystal violet-growth inhibition assay, click-iT 5-ethynyl-2′-deoxyuridine staining, and cell-cycle analysis. Biochemical assays were performed to study the underlying mechanism of drug treatment as a single agent or in combination with the MEK inhibitor trametinib. We further evaluated and compared the anti-tumor effects of single agent and combined treatment by using oral squamous cell carcinoma xenograft models.
Results
In this study, we showed that afatinib inhibited oral squamous cell carcinoma cell proliferation via cell-cycle arrest at the G0/G1 phase, and inhibited tumor growth in xenograft mouse models. Interestingly, we demonstrated reactivation of the mitogen-activated protein kinase (ERK1/2) pathway in vitro, which possibly reduced the effects of ErbB inhibition. Concomitant treatment of oral squamous cell carcinoma cells with afatinib and trametinib synergized the anti-tumor effects in oral squamous cell carcinoma-bearing mouse models.
Conclusions
Our findings provide insight into the molecular mechanism of resistance to afatinib and support further clinical evaluation into the combination of afatinib and MEK inhibition in the treatment of oral squamous cell carcinoma.





Similar content being viewed by others
References
Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58(3):903–13. https://doi.org/10.1016/j.ijrobp.2003.06.002.
Wheeler S, Siwak DR, Chai R, LaValle C, Seethala RR, Wang L, et al. Tumor epidermal growth factor receptor and EGFR PY1068 are independent prognostic indicators for head and neck squamous cell carcinoma. Clin Cancer Res. 2012;18(8):2278–89. https://doi.org/10.1158/1078-0432.CCR-11-1593.
Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90(11):824–32.
Saki M, Toulany M, Rodemann HP. Acquired resistance to cetuximab is associated with the overexpression of Ras family members and the loss of radiosensitization in head and neck cancer cells. Radiother Oncol. 2013;108(3):473–8. https://doi.org/10.1016/j.radonc.2013.06.023.
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–8. https://doi.org/10.1016/S1470-2045(09)70311-0.
Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA, Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(34):8646–54. https://doi.org/10.1200/jco.2005.02.4646.
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–27.
Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27(28):3944–56. https://doi.org/10.1038/onc.2008.19.
Zuo Q, Shi M, Li L, Chen J, Luo R. Development of cetuximab-resistant human nasopharyngeal carcinoma cell lines and mechanisms of drug resistance. Biomed Pharmacother. 2010;64(8):550–8. https://doi.org/10.1016/j.biopha.2010.03.003.
Madoz-Gurpide J, Zazo S, Chamizo C, Casado V, Carames C, Gavin E, et al. Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer. J Transl Med. 2015;13:282. https://doi.org/10.1186/s12967-015-0633-7.
Shimizu R, Ibaragi S, Eguchi T, Kuwajima D, Kodama S, Nishioka T, et al. Nicotine promotes lymph node metastasis and cetuximab resistance in head and neck squamous cell carcinoma. Int J Oncol. 2019;54(1):283–94. https://doi.org/10.3892/ijo.2018.4631.
Fujiwara T, Eguchi T, Sogawa C, Ono K, Murakami J, Ibaragi S, et al. Anti-EGFR antibody cetuximab is secreted by oral squamous cell carcinoma and alters EGF-driven mesenchymal transition. Biochem Biophys Res Commun. 2018;503(3):1267–72. https://doi.org/10.1016/j.bbrc.2018.07.035.
Ono K, Eguchi T, Sogawa C, Calderwood SK, Futagawa J, Kasai T, et al. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J Cell Biochem. 2018;119(9):7350–62. https://doi.org/10.1002/jcb.27039.
Fujiwara T, Eguchi T, Sogawa C, Ono K, Murakami J, Ibaragi S, et al. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncol. 2018;86:251–7. https://doi.org/10.1016/j.oraloncology.2018.09.030.
Lee BK, Tiong KH, Chang JK, Liew CS, Abdul Rahman ZA, Tan AC, et al. DeSigN: connecting gene expression with therapeutics for drug repurposing and development. BMC Genomics. 2017;18(Suppl. 1):934. https://doi.org/10.1186/s12864-016-3260-7.
Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–50.
Sos ML, Rode HB, Heynck S, Peifer M, Fischer F, Kluter S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR Inhibitors in tumor cells expressing the T790 M EGFR resistance mutation. Cancer Res. 2010;70(3):868–74. https://doi.org/10.1158/0008-5472.CAN-09-3106.
Seiwert T, Fayette J, Cupissol D, Del Campo J, Clement P, Hitt R, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25(9):1813–20. https://doi.org/10.1093/annonc/mdu216.
Machiels J-PH, Haddad RI, Fayette J, Licitra LF, Tahara M, Vermorken JB, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583–94. https://doi.org/10.1016/S1470-2045(15)70124-5.
Vermorken JB, Rottey S, Ehrnrooth E, Pelling K, Lahogue A, Wind S, et al. A phase Ib, open-label study to assess the safety of continuous oral treatment with afatinib in combination with two chemotherapy regimens: cisplatin plus paclitaxel and cisplatin plus 5-fluorouracil, in patients with advanced solid tumors. Ann Oncol. 2013;24(5):1392–400. https://doi.org/10.1093/annonc/mds633.
Chung CH, Rudek MA, Kang H, Marur S, John P, Tsottles N, et al. A phase I study afatinib/carboplatin/paclitaxel induction chemotherapy followed by standard chemoradiation in HPV-negative or high-risk HPV-positive locally advanced stage III/IVa/IVb head and neck squamous cell carcinoma. Oral Oncol. 2016;53:54–9. https://doi.org/10.1016/j.oraloncology.2015.11.020.
Perera SA, Li D, Shimamura T, Raso MG, Ji H, Chen L, et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci USA. 2009;106(2):474–9. https://doi.org/10.1073/pnas.0808930106.
Sun C, Hobor S, Bertotti A, Zecchin D, Huang S, Galimi F, et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transcriptional induction of ERBB3. Cell Rep. 2014;7(1):86–93. https://doi.org/10.1016/j.celrep.2014.02.045.
Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F, Ha SJ, et al. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790 M resistance mutation. Mol Cancer Ther. 2012;11(10):2254–64. https://doi.org/10.1158/1535-7163.MCT-12-0311.
Fadlullah MZ, Chiang IK, Dionne KR, Yee PS, Gan CP, Sam KK, et al. Genetically-defined novel oral squamous cell carcinoma cell lines for the development of molecular therapies. Oncotarget. 2016;7(19):27802–18. https://doi.org/10.18632/oncotarget.8533.
Holbeck SL, Collins JM, Doroshow JH. Analysis of food and drug administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol Cancer Ther. 2010;9(5):1451–60. https://doi.org/10.1158/1535-7163.MCT-10-0106.
Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81.
Schütze C, Dörfler A, Eicheler W, Zips D, Hering S, Solca F, et al. Combination of EGFR/HER2 tyrosine kinase inhibition by BIBW 2992 and BIBW 2669 with irradiation in FaDu human squamous cell carcinoma. Strahlenther Onkol. 2007;183(5):256–64.
Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119(10):3000–10.
Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac L, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–11.
Quesnelle KM, Grandis JR. Dual kinase inhibition of EGFR and HER2 overcomes resistance to cetuximab in a novel in vivo model of acquired cetuximab resistance. Clin Cancer Res. 2011;17(18):5935–44.
Young NR, Soneru C, Liu J, Grushko TA, Hardeman A, Olopade OI, et al. Afatinib efficacy against squamous cell carcinoma of the head and neck cell lines in vitro and in vivo. Target Oncol. 2015;10(4):501–8.
Yamaguchi K, Iglesias-Bartolome R, Wang Z, Callejas-Valera JL, Amornphimoltham P, Molinolo AA, et al. A synthetic-lethality RNAi screen reveals an ERK-mTOR co-targeting pro-apoptotic switch in PIK3CA + oral cancers. Oncotarget. 2016;7(10):10696–709. https://doi.org/10.18632/oncotarget.7372.
Sanceau J, Poupon M-F, Delattre O, Sastre-Garau X, Wietzerbin J. Strong inhibition of Ewing tumor xenograft growth by combination of human interferon-alpha or interferon-beta with ifosfamide. Oncogene. 2002;21(50):7700–9.
Zainal NS, Gan CP, Lau BF, Yee PS, Tiong KH, Abdul Rahman ZA, et al. Zerumbone targets the CXCR4-RhoA and PI3 K-mTOR signaling axis to reduce motility and proliferation of oral cancer cells. Phytomedicine. 2018;39:33–41. https://doi.org/10.1016/j.phymed.2017.12.011.
Huang W, Cui X, Chen J, Feng Y, Song E, Li J, et al. Long non-coding RNA NKILA inhibits migration and invasion of tongue squamous cell carcinoma cells via suppressing epithelial-mesenchymal transition. Oncotarget. 2016;7(38):62520–32. https://doi.org/10.18632/oncotarget.11528.
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6.
Takikita M, Xie R, Chung JY, Cho H, Ylaya K, Hong SM, et al. Membranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinoma. J Transl Med. 2011;9:126. https://doi.org/10.1186/1479-5876-9-126.
Ganly I, Talbot S, Carlson D, Viale A, Maghami E, Osman I, et al. Identification of angiogenesis/metastases genes predicting chemoradiotherapy response in patients with laryngopharyngeal carcinoma. J Clin Oncol. 2007;25(11):1369–76. https://doi.org/10.1200/JCO.2005.05.3397.
Yoshida T, Zhang G, Smith MA, Lopez AS, Bai Y, Li J, et al. Tyrosine phosphoproteomics identifies both codrivers and cotargeting strategies for T790 M-related EGFR-TKI resistance in non-small cell lung cancer. Clin Cancer Res. 2014;20(15):4059–74. https://doi.org/10.1158/1078-0432.CCR-13-1559.
Khelwatty SA, Essapen S, Seddon AM, Modjtahedi H. Growth response of human colorectal tumour cell lines to treatment with afatinib (BIBW2992), an irreversible erbB family blocker, and its association with expression of HER family members. Int J Oncol. 2011;39(2):483–91. https://doi.org/10.3892/ijo.2011.1054.
Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res. 2014;20(6):1410–6. https://doi.org/10.1158/1078-0432.CCR-13-1549.
Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–35. https://doi.org/10.1158/2159-8290.CD-11-0341.
Tricker EM, Xu C, Uddin S, Capelletti M, Ercan D, Ogino A, et al. Combined EGFR/MEK inhibition prevents the emergence of resistance in EGFR-mutant lung cancer. Cancer Discov. 2015;5(9):960–71. https://doi.org/10.1158/2159-8290.CD-15-0063.
Knowles LM, Stabile LP, Egloff AM, Rothstein ME, Thomas SM, Gubish CT, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15(11):3740–50. https://doi.org/10.1158/1078-0432.CCR-08-3252.
Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–28. https://doi.org/10.1038/nrc2536.
Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 2008;99(7):1319–25. https://doi.org/10.1111/j.1349-7006.2008.00840.x.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;2016(375):1856–67. https://doi.org/10.1056/NEJMoa1602252.
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–51. https://doi.org/10.1016/S0140-6736(15)60898-4.
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–9. https://doi.org/10.1056/NEJMoa1412690.
Witkiewicz AK, Balaji U, Eslinger C, McMillan E, Conway W, Posner B, et al. Integrated patient-derived models delineate individualized therapeutic vulnerabilities of pancreatic cancer. Cell Rep. 2016;16(7):2017–31. https://doi.org/10.1016/j.celrep.2016.07.023.
Manchado E, Weissmueller S, Morris JPT, Chen CC, Wullenkord R, Lujambio A, et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature. 2016;534(7609):647–51. https://doi.org/10.1038/nature18600.
Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–81. https://doi.org/10.1016/S1470-2045(12)70270-X.
Yang JCH, Reguart N, Barinoff J, Köhler J, Uttenreuther-Fischer M, Stammberger U, et al. Diarrhea associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther. 2013;13(6):729–36. https://doi.org/10.1586/era.13.31.
Cheng Y, Tian H. Current development status of MEK inhibitors. Molecules. 2017;22(10). pii: E1551. https://doi.org/10.3390/molecules22101551.
Acknowledgements
The authors thank Drs. J. Silvio Gutkind and Wang Zhiyong for sharing the CAL27 cells. The authors also thank all the staff of the animal facility at the National University of Malaysia (Universiti Kebangsaan Malaysia), Bangi, Malaysia for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by High Impact Research, Ministry of Higher Education (HIR-MOHE) from the University of Malaya (UM.C/625/1/HIR/MOHE/DENT-03) and other sponsors of Cancer Research Malaysia. Cancer Research Malaysia is a non-profit research organization committed to an understanding of cancer prevention, diagnosis, and treatment through a fundamental research program.
Conflict of interest
Pei San Yee, Nur Syafinaz Zainal, Chai Phei Gan, Bernard K.B. Lee, Kein Seong Mun, Mannil Thomas Abraham, Siti Mazlipah Ismail, Zainal Ariff Abdul Rahman, Vyomesh Patel, and Sok Ching Cheong have no conflicts of interest that are directly relevant to the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yee, P.S., Zainal, N.S., Gan, C.P. et al. Synergistic Growth Inhibition by Afatinib and Trametinib in Preclinical Oral Squamous Cell Carcinoma Models. Targ Oncol 14, 223–235 (2019). https://doi.org/10.1007/s11523-019-00626-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00626-8