Abstract
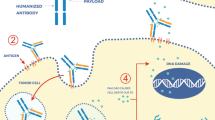
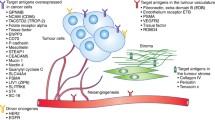
Antibody–drug conjugates (ADCs) are complex immunoconjugates designed to selectively deliver toxic small molecules preferentially to cancer cells. These immunoconjugates consist of a monoclonal antibody - directed to a tumor antigen - and a cytotoxic agent that is conjugated to the antibody via a molecular linker. Following the binding to a specific antigen on the surface of cancer cells, the conjugate is internalized and releases its cytotoxic payload to kill the malignant cell. ADCs that have gained regulatory approval from the US Food and Drug Administration (FDA) include brentuximab vedotin for CD30-positive Hodgkin’s lymphoma and trastuzumab emtansine for human epidermal growth factor receptor 2 (HER2)-positive breast cancer. Several other agents are in advanced stages of clinical development, including sacituzumab govitecan for breast cancer, mirvetuximab soravtansine for ovarian cancer, rovalpituzumab tesirine for lung cancer, depatuxizumab mafodotin for glioblastoma, and oportuzumab monatox for bladder cancer. This review provides an overview of the recent clinical experience with the approved, most advanced, and other promising candidates of ADCs for solid tumors, including a description of biology and chemistry of ADCs, drug resistance and biomarkers, and the future perspective on combination strategies with these new immunoconjugates.




Similar content being viewed by others
References
Goodman LS, Wintrobe MM, et al. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin's disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc. 1946;132:126–32.
Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238(23):787–93. https://doi.org/10.1056/NEJM194806032382301.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7.
Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15(6):361–70. https://doi.org/10.1038/nrc3930.
Schwartz RS. Paul Ehrlich's magic bullets. N Engl J Med. 2004;350(11):1079–80. https://doi.org/10.1056/NEJMp048021.
Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014;6(1):34–45. https://doi.org/10.4161/mabs.27022.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–54. https://doi.org/10.1200/JCO.2001.19.13.3244.
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7(6):1490–6.
Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–6. https://doi.org/10.1200/JCO.2011.38.0402.
Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–9. https://doi.org/10.1200/JCO.2011.38.0410.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. https://doi.org/10.1056/NEJMoa1209124.
Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6(9):714–27. https://doi.org/10.1038/nrc1913.
Patil R, Portilla-Arias J, Ding H, Konda B, Rekechenetskiy A, Inoue S, et al. Cellular delivery of doxorubicin via pH-controlled hydrazone linkage using multifunctional nano vehicle based on poly(beta-l-malic acid). Int J Mol Sci. 2012;13(9):11681–93. https://doi.org/10.3390/ijms130911681.
Jain N, Smith SW, Ghone S, Tomczuk B. Current ADC linker chemistry. Pharm Res. 2015;32(11):3526–40. https://doi.org/10.1007/s11095-015-1657-7.
Polson AG, Calemine-Fenaux J, Chan P, Chang W, Christensen E, Clark S, et al. Antibody-drug conjugates for the treatment of non-Hodgkin's lymphoma: target and linker-drug selection. Cancer Res. 2009;69(6):2358–64. https://doi.org/10.1158/0008-5472.CAN-08-2250.
Smith AL, Nicolaou KC. The enediyne antibiotics. J Med Chem. 1996;39(11):2103–17. https://doi.org/10.1021/jm9600398.
David M, Goldenberg TMC, Serengulam V, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–512.
Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6(10):789–802. https://doi.org/10.1038/nrc1977.
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–65. https://doi.org/10.1182/blood-2003-01-0039.
Li F, Emmerton KK, Jonas M, Zhang X, Miyamoto JB, Setter JR, et al. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res. 2016;76(9):2710–9. https://doi.org/10.1158/0008-5472.CAN-15-1795.
Hinrichs MJ, Dixit R. Antibody drug conjugates: nonclinical safety considerations. AAPS J. 2015;17(5):1055–64. https://doi.org/10.1208/s12248-015-9790-0.
Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs. 2016;8(4):659–71. https://doi.org/10.1080/19420862.2016.1156829.
Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35(4):e00225–e. https://doi.org/10.1042/bsr20150089.
Tolcher AW, Sugarman S, Gelmon KA, Cohen R, Saleh M, Isaacs C, et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17(2):478–84. https://doi.org/10.1200/JCO.1999.17.2.478.
Gorovits B, Krinos-Fiorotti C. Proposed mechanism of off-target toxicity for antibody-drug conjugates driven by mannose receptor uptake. Cancer Immunol Immunother. 2013;62(2):217–23. https://doi.org/10.1007/s00262-012-1369-3.
Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–58. https://doi.org/10.1016/j.addr.2007.06.008.
Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol. 2009;13(3):235–44. https://doi.org/10.1016/j.cbpa.2009.03.023.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. https://doi.org/10.1038/nri2155.
Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. https://doi.org/10.3389/fonc.2012.00062.
Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115–23. https://doi.org/10.1053/j.seminhematol.2010.01.011.
Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–75. https://doi.org/10.1038/nrc2656.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. https://doi.org/10.1038/35052073.
Weinberg RA. Twisted epithelial-mesenchymal transition blocks senescence. Nat Cell Biol. 2008;10(9):1021–3. https://doi.org/10.1038/ncb0908-1021.
Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61(12):4744–9.
Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27(34):5838–47. https://doi.org/10.1200/JCO.2009.22.1507.
Bardia A, Baselga J. Targeted Therapies in Breast Cancer. In: Sledge G, Baselga J, editors. Targeted therapies in breast cancer (therapeutic strategies). 1st ed. Oxford: Clinical Publishing; 2013. p. 43–50.
Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–27. https://doi.org/10.1038/nri2744.
Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. https://doi.org/10.1038/cr.2009.139.
Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–40. https://doi.org/10.1038/nri2620.
Stoermer KA, Morrison TE. Complement and viral pathogenesis. Virology. 2011;411(2):362–73. https://doi.org/10.1016/j.virol.2010.12.045.
Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85(1):1–14.
Al-Shamkhani A. The role of CD30 in the pathogenesis of haematopoietic malignancies. Curr Opin Pharmacol. 2004;4(4):355–9. https://doi.org/10.1016/j.coph.2004.02.007.
Matsumoto K, Terakawa M, Miura K, Fukuda S, Nakajima T, Saito H. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004;172(4):2186–93.
Wahl AF, Klussman K, Thompson JD, Chen JH, Francisco LV, Risdon G, et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin's disease. Cancer Res. 2002;62(13):3736–42.
Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171–9. https://doi.org/10.1111/j.1365-2141.2009.07740.x.
Sutherland MS, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–7. https://doi.org/10.1074/jbc.M510026200.
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16(3):888–97. https://doi.org/10.1158/1078-0432.CCR-09-2069.
Chen R, Hou J, Newman E, Kim Y, Donohue C, Liu X, et al. CD30 Downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol Cancer Ther. 2015;14(6):1376–84. https://doi.org/10.1158/1535-7163.MCT-15-0036.
Oflazoglu E, Stone IJ, Gordon KA, Grewal IS, van Rooijen N, Law CL, et al. Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood. 2007;110(13):4370–2. https://doi.org/10.1182/blood-2007-06-097014.
Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071–9. https://doi.org/10.1093/annonc/mdm090.
Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–62. https://doi.org/10.1016/S0140-6736(15)60165-9.
O'Connor OA. Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182–9. https://doi.org/10.1200/JCO.2010.29.9024.
Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390(10094):555–66. https://doi.org/10.1016/S0140-6736(17)31266-7.
Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5(7):953–62.
Krop I, Winer EP. Trastuzumab emtansine: a novel antibody-drug conjugate for HER2-positive breast cancer. Clin Cancer Res. 2014;20(1):15–20. https://doi.org/10.1158/1078-0432.CCR-13-0541.
Oroudjev E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, et al. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol Cancer Ther. 2010;9(10):2700–13. https://doi.org/10.1158/1535-7163.MCT-10-0645.
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90. https://doi.org/10.1158/0008-5472.CAN-08-1776.
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128(2):347–56. https://doi.org/10.1007/s10549-010-1090-x.
Krop IE, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–99. https://doi.org/10.1016/S1470-2045(14)70178-0.
Harbeck N, Gluz O, Christgen M, Kates RE, Braun M, Kuemmel S, et al. De-escalation strategies in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer (BC): final analysis of the west German study group adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early BC HER2- and hormone receptor-positive phase II randomized trial-efficacy, safety, and predictive markers for 12 weeks of Neoadjuvant Trastuzumab Emtansine with or without endocrine therapy (ET) versus Trastuzumab plus ET. J Clin Oncol. 2017;35(26):3046–54. https://doi.org/10.1200/JCO.2016.71.9815.
Phillips GD, Fields CT, Li G, Dowbenko D, Schaefer G, Miller K, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. 2014;20(2):456–68. https://doi.org/10.1158/1078-0432.CCR-13-0358.
Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141–8.
Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17(1):1–12. https://doi.org/10.1007/s10120-013-0252-z.
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–84. https://doi.org/10.1007/s10120-014-0402-y.
Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53. https://doi.org/10.1016/S1470-2045(17)30111-0.
Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50. https://doi.org/10.1038/modpathol.2011.198.
Peng Z, Zou J, Zhang X, Yang Y, Gao J, Li Y, et al. HER2 discordance between paired primary gastric cancer and metastasis: a meta-analysis. Chin J Cancer Res. 2015;27(2):163–71. https://doi.org/10.3978/j.issn.1000-9604.2014.12.09.
Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):209. https://doi.org/10.1186/bcr3621.
Loganzo F, Tan X, Sung M, Jin G, Myers JS, Melamud E, et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther. 2015;14(4):952–63. https://doi.org/10.1158/1535-7163.MCT-14-0862.
Sendur MA, Aksoy S, Altundag K. Cardiotoxicity of novel HER2-targeted therapies. Curr Med Res Opin. 2013;29(8):1015–24. https://doi.org/10.1185/03007995.2013.807232.
Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN, et al. Efficacy and safety of anti-Trop-2 antibody drug conjugate Sacituzumab Govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol. 2017;35(19):2141–8. https://doi.org/10.1200/JCO.2016.70.8297.
Heist RS, Guarino MJ, Masters G, Purcell WT, Starodub AN, Horn L, et al. Therapy of advanced non-small-cell lung cancer with an SN-38-anti-Trop-2 drug conjugate, Sacituzumab Govitecan. J Clin Oncol. 2017;35(24):2790–7. https://doi.org/10.1200/JCO.2016.72.1894.
Faltas B, Goldenberg DM, Ocean AJ, Govindan SV, Wilhelm F, Sharkey RM, et al. Sacituzumab Govitecan, a novel antibody--drug conjugate, in patients with metastatic platinum-resistant Urothelial carcinoma. Clin Genitourin Cancer. 2016;14(1):e75–9. https://doi.org/10.1016/j.clgc.2015.10.002.
Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, Forero A, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate Glembatumumab Vedotin in advanced glycoprotein NMB-expressing breast cancer. J Clin Oncol. 2015;33(14):1609–19. https://doi.org/10.1200/JCO.2014.56.2959.
Ott PA, Hamid O, Pavlick AC, Kluger H, Kim KB, Boasberg PD, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J Clin Oncol. 2014;32(32):3659–66. https://doi.org/10.1200/JCO.2013.54.8115.
Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A. 1981;78(8):5147–50.
Ripani E, Sacchetti A, Corda D, Alberti S. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76(5):671–6.
Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011;17(10):3157–69. https://doi.org/10.1158/1078-0432.CCR-10-2939.
Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32(2):222–33. https://doi.org/10.1038/onc.2012.36.
Lin H, Huang JF, Qiu JR, Zhang HL, Tang XJ, Li H, et al. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp Mol Pathol. 2013;94(1):73–8. https://doi.org/10.1016/j.yexmp.2012.08.004.
Liu LF, Desai SD, Li TK, Mao Y, Sun M, Sim SP. Mechanism of action of camptothecin. Ann N Y Acad Sci. 2000;922:1–10.
Xu Y. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol. 2002;13(12):1841–51. https://doi.org/10.1093/annonc/mdf337.
Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Arrojo R, Liu D, et al. Sacituzumab Govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26(5):919–31. https://doi.org/10.1021/acs.bioconjchem.5b00223.
Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res. 2001;7(8):2182–94.
Weterman MA, Ajubi N, van Dinter IM, Degen WG, van Muijen GN, Ruitter DJ, et al. Nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995;60(1):73–81.
Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol. 2007;178(10):6557–66.
Abdelmagid SM, Barbe MF, Rico MC, Salihoglu S, Arango-Hisijara I, Selim AH, et al. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Exp Cell Res. 2008;314(13):2334–51. https://doi.org/10.1016/j.yexcr.2008.02.006.
Selim AA, Abdelmagid SM, Kanaan RA, Smock SL, Owen TA, Popoff SN, et al. Anti-osteoactivin antibody inhibits osteoblast differentiation and function in vitro. Crit Rev Eukaryot Gene Expr. 2003;13(2–4):265–75.
Rose AA, Grosset AA, Dong Z, Russo C, Macdonald PA, Bertos NR, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16(7):2147–56. https://doi.org/10.1158/1078-0432.CCR-09-1611.
Kuan CT, Wakiya K, Dowell JM, Herndon JE 2nd, Reardon DA, Graner MW, et al. Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin Cancer Res. 2006;12(7 Pt 1):1970–82. https://doi.org/10.1158/1078-0432.CCR-05-2797.
Rich JN, Shi Q, Hjelmeland M, Cummings TJ, Kuan CT, Bigner DD, et al. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. J Biol Chem. 2003;278(18):15951–7. https://doi.org/10.1074/jbc.M211498200.
Rose AA, Annis MG, Dong Z, Pepin F, Hallett M, Park M, et al. ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PLoS One. 2010;5(8):e12093. https://doi.org/10.1371/journal.pone.0012093.
Tse KF, Jeffers M, Pollack VA, McCabe DA, Shadish ML, Khramtsov NV, et al. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12(4):1373–82. https://doi.org/10.1158/1078-0432.CCR-05-2018.
Pollack VA, Alvarez E, Tse KF, Torgov MY, Xie S, Shenoy SG, et al. Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemother Pharmacol. 2007;60(3):423–35. https://doi.org/10.1007/s00280-007-0490-z.
Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet. 2011;20(5):905–16. https://doi.org/10.1093/hmg/ddq529.
Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139(23):4365–73. https://doi.org/10.1242/dev.083840.
Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12(5):535–42. https://doi.org/10.1634/theoncologist.12-5-535.
Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7(302):302ra136. https://doi.org/10.1126/scitranslmed.aac9459.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51. https://doi.org/10.1016/S1470-2045(16)30565-4.
Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56(8):1067–84. https://doi.org/10.1016/j.addr.2004.01.001.
Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26(1):141–52. https://doi.org/10.1007/s10555-007-9048-0.
Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26(10):2034–43. https://doi.org/10.1093/annonc/mdv250.
Toffoli G, Russo A, Gallo A, Cernigoi C, Miotti S, Sorio R, et al. Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer. Int J Cancer. 1998;79(2):121–6.
Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108(3):619–26. https://doi.org/10.1016/j.ygyno.2007.11.020.
Widdison WC, Wilhelm SD, Cavanagh EE, Whiteman KR, Leece BA, Kovtun Y, et al. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem. 2006;49(14):4392–408. https://doi.org/10.1021/jm060319f.
Ab O, Whiteman KR, Bartle LM, Sun X, Singh R, Tavares D, et al. IMGN853, a Folate receptor-alpha (FRalpha)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRalpha-expressing Tumors. Mol Cancer Ther. 2015;14(7):1605–13. https://doi.org/10.1158/1535-7163.MCT-14-1095.
Moore KN, Martin LP, O'Malley DM, Matulonis UA, Konner JA, Perez RP, et al. Safety and activity of Mirvetuximab Soravtansine (IMGN853), a Folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol. 2017;35(10):1112–8. https://doi.org/10.1200/JCO.2016.69.9538.
Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33(4):369–85. https://doi.org/10.1053/j.seminoncol.2006.04.003.
Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19(11):1389–400. https://doi.org/10.1038/nm.3388.
Gan HK, Burgess AW, Clayton AH, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72(12):2924–30. https://doi.org/10.1158/0008-5472.CAN-11-3898.
Gan HK, Burge ME, Solomon BJ, Holen KD, Zhang Y, Ciprotti M, et al. A phase I and biodistribution study of ABT-806i, an 111indium-labeled conjugate of the tumor-specific anti-EGFR antibody ABT-806. J Clin Oncol. 2013;31(15_suppl):2520. https://doi.org/10.1200/jco.2013.31.15_suppl.2520.
Phillips AC, Boghaert ER, Vaidya KS, Mitten MJ, Norvell S, Falls HD, et al. ABT-414, an antibody-drug conjugate targeting a tumor-selective EGFR Epitope. Mol Cancer Ther. 2016;15(4):661–9. https://doi.org/10.1158/1535-7163.MCT-15-0901.
Gan HK, Fichtel L, Lassman A, Merrell R, Bent M, Kumthekar P. A phase I study evaluating ABT414 with concurrent radiotherapy (RT) and temozolomide (TMZ) in glioblastoma (GBM) [abstract no. ET-19]. Neuro Oncol. 2014;16(Suppl 5):v83.
Reardon DA, Lassman AB, van den Bent M, Kumthekar P, Merrell R, Scott AM, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol. 2017 Jul 1;19(7):965–75. https://doi.org/10.1093/neuonc/now257.
Di Paolo C, Willuda J, Kubetzko S, Lauffer I, Tschudi D, Waibel R, et al. A recombinant immunotoxin derived from a humanized epithelial cell adhesion molecule-specific single-chain antibody fragment has potent and selective antitumor activity. Clin Cancer Res. 2003;9(7):2837–48.
Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM). J Mol Med (Berl). 1999;77(10):699–712.
Oppenheimer NJ, Bodley JW. Diphtheria toxin. Site and configuration of ADP-ribosylation of diphthamide in elongation factor 2. J Biol Chem. 1981;256(16):8579–81.
Perentesis JP, Miller SP, Bodley JW. Protein toxin inhibitors of protein synthesis. Biofactors. 1992;3(3):173–84.
Kowalski M, Guindon J, Brazas L, Moore C, Entwistle J, Cizeau J, et al. A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guerin. J Urol. 2012;188(5):1712–8. https://doi.org/10.1016/j.juro.2012.07.020.
Manning DL, Daly RJ, Lord PG, Kelly KF, Green CD. Effects of oestrogen on the expression of a 4.4 kb mRNA in the ZR-75-1 human breast cancer cell line. Mol Cell Endocrinol. 1988;59(3):205–12.
Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375(Pt 1):51–9. https://doi.org/10.1042/BJ20030478.
Taylor KM, Hiscox S, Nicholson RI. Zinc transporter LIV-1: a link between cellular development and cancer progression. Trends Endocrinol Metab. 2004;15(10):461–3. https://doi.org/10.1016/j.tem.2004.10.003.
Sussman D, Smith LM, Anderson ME, Duniho S, Hunter JH, Kostner H, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014;13(12):2991–3000. https://doi.org/10.1158/1535-7163.MCT-13-0896.
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA Topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–108. https://doi.org/10.1158/1078-0432.CCR-15-2822.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. https://doi.org/10.1111/cas.12966.
Shiose Y, Ochi Y, Kuga H, Yamashita F, Hashida M. Relationship between drug release of DE-310, macromolecular prodrug of DX-8951f, and cathepsins activity in several tumors. Biol Pharm Bull. 2007;30(12):2365–70.
Nakada T, Masuda T, Naito H, Yoshida M, Ashida S, Morita K, et al. Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg Med Chem Lett. 2016;26(6):1542–5. https://doi.org/10.1016/j.bmcl.2016.02.020.
Bergstrom D, Bodyak N, Park P, Yurkovetskiy A, DeVit M, Yin M, et al. XMT-1522 induces tumor regressions in pre-clinical models representing HER2-positive and HER2 low-expressing breast cancer [abstract no. P4-14-28]. Cancer Res. 2016;76(4 Suppl):P4-14-28-P4-14-28. https://doi.org/10.1158/1538-7445.sabcs15-p4-14-28.
Hamilton GS. Antibody-drug conjugates for cancer therapy: the technological and regulatory challenges of developing drug-biologic hybrids. Biologicals. 2015;43(5):318–32. https://doi.org/10.1016/j.biologicals.2015.05.006.
Baselga J, Lewis Phillips GD, Verma S, Ro J, Huober J, Guardino AE, et al. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of Trastuzumab Emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res. 2016;22(15):3755–63. https://doi.org/10.1158/1078-0432.CCR-15-2499.
Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32(33):3753–61. https://doi.org/10.1200/JCO.2013.54.5384.
Ritchie M, Tchistiakova L, Scott N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs. 2013;5(1):13–21. https://doi.org/10.4161/mabs.22854.
Muller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7(315):315ra188. https://doi.org/10.1126/scitranslmed.aac4925.
Cardillo TM, Sharkey RM, Rossi DL, Arrojo R, Mostafa AA, Goldenberg DM. Synthetic lethality exploitation by an anti-Trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin Cancer Res. 2017 Jul 1;23(13):3405–15. https://doi.org/10.1158/1078-0432.CCR-16-2401.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
A. Bardia was supported by the US National Institutes of Health (NIH) (K12CA087723–14).
Conflict of Interest
Aiko Nagayama owns stock options in Chugai, Inc., and a family member has a leadership position with Chugai, Inc. and Roche, Inc. Bruce Chabner owns stocks in Seattle Genetics. Aditya Bardia has received consulting fees or honorarium by Genentech, Pfizer, and Novartis. Leif Ellisen declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Nagayama, A., Ellisen, L.W., Chabner, B. et al. Antibody–Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments. Targ Oncol 12, 719–739 (2017). https://doi.org/10.1007/s11523-017-0535-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0535-0