Abstract
Background
If patients in oncology trials receive subsequent therapy, standard intention-to-treat (ITT) analyses may inaccurately estimate the overall survival (OS) effect of the investigational product. In this context, a post-hoc analysis of the phase 3 PREVAIL study was performed with the aim to compare enzalutamide with placebo in terms of OS, adjusting for potential confounding from switching to antineoplastic therapies that are not part of standard metastatic castration-resistant prostate cancer (mCRPC) treatment pathways in some jurisdictions.
Methods
The PREVAIL study, which included 1717 chemotherapy-naïve men with mCRPC randomized to treatment with enzalutamide 160 mg/day or placebo, was stopped after a planned interim survival analysis revealed a benefit in favor of enzalutamide. Data from this cutoff point were confounded by switching from both arms and so were evaluated in terms of OS using two switching adjustment methods: the two-stage accelerated failure time model (two-stage method) and inverse probability of censoring weights (IPCW).
Results
Following adjustment for switching to nonstandard antineoplastic therapies by 14.8 (129/872 patients) and 21.3% (180/845 patients) of patients initially randomized to enzalutamide and placebo, respectively, the two-stage and IPCW methods both resulted in numerical reductions in the hazard ratio (HR) for OS [HR 0.66, 95% confidence interval (CI) 0.57–0.81 and HR 0.63, 95% CI 0.52–0.75, respectively] for enzalutamide compared to placebo versus the unadjusted ITT analysis (HR 0.71, 95% CI 0.60–0.84). These results suggest a slightly greater effect of enzalutamide on OS than originally reported.
Conclusion
In the PREVAIL study, switching to nonstandard antineoplastic mCRPC therapies resulted in the ITT analysis of primary data underestimating the benefit of enzalutamide on OS.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
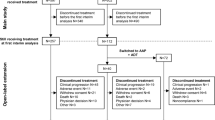
Treatment switching has become an important issue in the development and approval of new drugs, particularly with regard to oncology [1]. In oncology trials, if cancer progresses, it is common to offer patients the option of switching from their randomized treatment to another active therapy (Fig. 1). Although the practice of treatment switching has the potential to affect the results of clinical evaluations of investigational products, it is likely to be more problematic for health technology assessment (HTA) agencies than for licensing bodies. For the purpose of regulatory approval, an accurate estimate of the overall survival (OS) hazard ratio (HR) may not always be required, and in some instances a statistically significant advantage for progression-free survival (PFS) may be sufficient. However, for an HTA, accurate estimates of both the PFS and OS treatment effect are needed; therefore, treatment switching is more of an issue.
Importantly, if patients switch to and benefit from another active therapy, the true OS benefit associated with the original investigational product may be inaccurately estimated using standard intention-to-treat (ITT) methodology, and this in turn can affect subsequent cost-effectiveness analyses that use OS evidence [2]. Adjustment methods are available that provide better estimates of true drug effects. However, economic evaluations should only adjust for switching to active postprogression therapies that are not part of the standard treatment pathway [2]. From a pragmatic perspective, decision-makers typically want to ascertain the effect of including a new drug in a therapeutic regimen. If a new treatment improves the prognosis of a patient and enhances their suitability for subsequent treatment lines, this can be considered as a benefit of treatment for which adjustments are unnecessary, provided the subsequent treatments reflect those that would be expected in reality. However, if patients switch to poststudy experimental or nonstandard therapies, the survival experience observed in the trial will not be that which would be expected in reality; for these switches, adjustments are required to estimate the expected impact of adding the new treatment into the standard treatment pathway.
A range of switching adjustment methods are available, and these are generally classified as randomization based, such as the rank-preserving structural failure time model (RPSFTM) [3] or iterative parameter estimation algorithm (IPE) [4]), or observational based, such as the two-stage accelerated failure time model (two-stage method) [5, 6] or inverse probability of censoring weights (IPCW) [7]).
As different switching adjustment methods have varying limitations, they are appropriate in different scenarios, and the most adequate method depends on the characteristics of the trial from which data are being evaluated [2].
Enzalutamide is a potent oral androgen-receptor signaling inhibitor developed to treat metastatic castration-resistant prostate cancer (mCRPC) [8]. Its clinical benefits have been demonstrated in the phase 3, double-blind, placebo-controlled AFFIRM and PREVAIL studies [9, 10]. Results of the AFFIRM study [9] supported the initial approval of enzalutamide to treat mCRPC that had progressed after chemotherapy, and those of the PREVAIL study [10] supported extension of the indication to include patients who were chemotherapy naïve [11, 12].
PREVAIL was stopped after a planned interim survival analysis (data cutoff September 16, 2013), performed at 540 reported deaths, which revealed a benefit in favor of enzalutamide for radiographic PFS [ITT analysis HR for enzalutamide vs. placebo 0.19; 95% confidence interval (CI) 0.15–0.23; P < 0.001] and OS (ITT analysis HR for enzalutamide vs. placebo 0.71; 95% CI 0.60–0.84; P < 0.001) [10]. All patients were followed up for OS after discontinuing study treatment. At the time of the September 2013 data cutoff, 43.8% (382/872) of patients in the enzalutamide arm and 76% (642/845) of patients in the placebo arm had received subsequent antineoplastic therapy (40.3 and 70.3%, respectively, had taken subsequent antineoplastics associated with a demonstrated survival benefit in metastatic prostate cancer) [10].
Here we describe analyses that estimate the effects of enzalutamide versus placebo on OS, adjusting for the potential confounding effects of switching to antineoplastic therapies that are not part of the standard mCRPC treatment pathway in the UK. Although the analyses are therefore presented from a UK perspective, where such analyses are routinely considered by the National Institute for Health and Care Excellence (NICE) [13], adjustment analyses are becoming more commonly expected in other jurisdictions [1]. With due consideration of standard treatment pathways in other countries, adjustment analyses could be tailored accordingly.
2 Methods
2.1 Study Design
This is a post-hoc analysis of OS following treatment switching in patients enrolled in the PREVAIL study. PREVAIL (ClinicalTrials.gov number: NCT01212991) included 1717 chemotherapy-naïve men with mCRPC who were randomized to receive enzalutamide 160 mg/day (n = 872) or placebo (n = 845) [10]. OS and PFS were coprimary endpoints.
In our analysis, treatment switching was defined as the receipt of any postbaseline antineoplastic therapy, excluding treatment to which patients were randomized, that was not part of the standard treatment pathway in the UK according to the NICE clinical guideline for prostate cancer [14] and clinical expert opinion. Nonstandard treatment comprised enzalutamide, abiraterone, cabazitaxel, sipuleucel-T and investigational drugs, as well as other chemotherapeutic cytotoxic drugs (including estramustine, cyclophosphamide, mitoxantrone, carboplatin, cisplatin and chemotherapeutics) and noncytotoxic treatment (including cetuximab, methotrexate, and thalidomide). Patients receiving such nonstandard therapy were termed switchers. Patients receiving standard treatment (i.e. docetaxel, hormonal treatments or antiandrogens) or who did not receive any other antineoplastic treatment after study drug discontinuation, were regarded to be nonswitchers.
Various statistical methods are available to adjust survival estimates in the presence of treatment switching [15]. Following recommendations in the NICE Decision Support Unit Technical Support Document 16 [15], we evaluated the characteristics of PREVAIL [9] and treatment switching mechanism and assessed the applicability and suitability of the different treatment switching adjustment methods. Based on the results of this assessment, we considered two observational-based methods, namely, the two-stage method and the IPCW, to be the most appropriate for use in the current analysis.
2.2 Adjustment Methods
As the two-stage and IPCW methods have been described and their use considered previously [5–7, 15], these are only described briefly here. More in-depth information relating to the application of these methods to the PREVAIL data is provided in Electronic Supplementary Material (ESM) 1.
Both methods included the same covariates (Table 1), with selection based on clinical expert opinion and prior knowledge of the disease. Missing values for covariates were imputed using either the mean for the trial population or the last observation carried forward. Parameter estimate values and odds ratios for the coefficients for the two-stage (Weibull and generalized gamma applications) and IPCW methods (September 2013 data cutoff) have been included in ESM 2–5. HR and 95% CI data for the IPCW-adjusted Cox proportional hazards model are provided in ESM 6.
2.2.1 Two-Stage Method
In general terms, the two-stage method involves estimating a treatment effect specific to switching patients and using this effect to derive a counterfactual dataset unaffected by switching [5, 6]. A disease event, defined here as the treatment discontinuation date, is used as a secondary baseline, and data beyond this point are treated as an observational dataset. As both the enzalutamide and placebo arms of PREVAIL included switchers, data from the secondary baseline onward were regarded as two separate observational datasets—one for each randomized treatment group. Accelerated failure time models (Weibull and generalized gamma) were fitted separately to the two observational datasets to estimate the treatment effect associated with switching in the two randomized groups. Acceleration factors (AFs), calculated separately for the enzalutamide and placebo arms, were used to shrink the survival time for switchers in each group to derive the counterfactual survival dataset—that is, a dataset in which survival times of switching patients were adjusted to those times that would have been expected had switching not occurred. Standard survival analyses were performed on this dataset to estimate the treatment effect of enzalutamide versus placebo, adjusted to account for treatment switching. The fits of the accelerated failure time models were evaluated using the Akaike information criterion (AIC) and Bayesian Information Criterion (BIC), with lower values indicative of a better statistical fit.
With the methodology involving estimation of counterfactual survival times, recensoring was undertaken as recommended in the literature [16]. Results without recensoring were also assessed because recensoring can lead to bias if the treatment effect varies over time [15].
Finally, a Cox regression model was used to estimate an adjusted HR for enzalutamide on OS, and the 95% CI was estimated by bootstrapping the entire adjustment process.
This two-stage method can only be used if an appropriate secondary baseline exists, at which point there should be no unmeasured confounders, and switching should occur promptly after the secondary baseline to avoid time-dependent confounding.
2.2.2 IPCW Method
The IPCW method as applied here involved censoring patients at the time of treatment switch and weighting the follow-up information for the remaining patients so that the information accounted both for the remaining patients and for patients with similar characteristics (both baseline and time dependent) whose follow-up was censored [7]. A weighted Cox regression model was used to estimate an adjusted HR for OS, and a 95% CI for the HR was estimated using bootstrapping. A weighted Kaplan–Meier curve was also obtained.
The IPCW method assumes that there are no unmeasured confounders, with data being available for all baseline and time-dependent prognostic factors for mortality that also predict informative censoring (switching). The method also relies on the models for switching and survival being correctly specified. Stabilized weights are commonly used within the IPCW method [7]. When switching proportions are very high, or if certain patient characteristics are very strong predictors of switching, extreme weights can be obtained. In these circumstances the IPCW becomes prone to error [5, 6]; to investigate this, we analyzed the weights obtained.
3 Results
The results presented below focus on the analysis of data obtained during the PREVAIL trial at the cutoff at which the planned interim survival analysis, performed at 540 reported deaths, revealed a benefit in favor of enzalutamide (September 16, 2013) [9]. However, when granting the extended indication for enzalutamide, the US Food and Drug Administration (FDA) requested an updated analysis of the OS endpoint to be performed that contained a minimum of the original protocol-defined number of deaths (765); the data cutoff for this subsequent analysis was June 1, 2014 [17]. Consequently, results pertaining to the latter cutoff are also briefly considered at the end of the Results section.
3.1 Patient Disposition and Treatment Switching
At the interim data cutoff (September 16, 2013), treatment with the study medication was ongoing for 42.1% of patients randomized to receive enzalutamide (367/872) and 7.2% of those assigned to placebo (61/845) (Table 2). There was one patient in each group who did not receive study treatment. Thus, a total of 1287 patients discontinued the study drug, of whom more were in the placebo arm (92.7%, 783/845) than in the enzalutamide arm (57.8%, 504/872). The primary reason for study drug discontinuation was disease progression, reported for 70.4% (355/504) and 73.7% (577/783) of discontinuing patients in the enzalutamide and placebo arms, respectively.
A total of 309 switchers were identified (Table 2); of these, 14.8% (129/872) and 21.3% (180/845) of patients in the enzalutamide arm and placebo arm, respectively, received antineoplastic therapies that were not part of the standard treatment pathway in the UK. The most common of the nonstandard subsequent antineoplastic therapies received first upon study treatment discontinuation was abiraterone, which was administered to 47.3% (61/129) and 50% (90/180) of enzalutamide and placebo switchers, respectively.
Docetaxel was administered to 26.1% (228/872) of patients in the enzalutamide arm and 47.5% (401/845) of patients in the placebo arm as the first antineoplastic therapy after study drug discontinuation (Table 3). Given that docetaxel is part of the standard treatment pathway in the UK, these patients were classified as nonswitchers in our analysis.
The mean (median) time from study drug discontinuation to the start of first antineoplastic treatment was 2.15 (1.28) months for the overall population, with similar distributions in the enzalutamide arm and placebo arm [1.98 (1.25) vs. 2.28 (1.28) months, respectively].
There were potentially important differences in the demographic characteristics of switchers and nonswitchers. Higher proportions of switchers than nonswitchers were asymptomatic at baseline [69.2% (211/305) vs. 62.5% (606/970), respectively] and had an Eastern Cooperative Oncology Group Performance Status of 0 at the last assessment before treatment discontinuation [58.9% (182/309) vs. 38.7% (380/981), respectively], while higher proportions of nonswitchers than switchers experienced grade 3 or greater adverse events at the last assessment before treatment discontinuation [27.9% (274/981) vs. 14.6% (45/309), respectively] or at any time prior to this [41.4% (406/981) vs. 25.2% (78/309), respectively].
3.2 Overall Survival
3.2.1 Unadjusted ITT Analysis
The results of an unadjusted ITT analysis suggested that enzalutamide extended OS relative to placebo (HR 0.71; 95% CI 0.60–0.84).
3.2.2 Two-Stage Method
For the two-stage method, AFs were calculated by fitting generalized gamma and Weibull models to the observational datasets (Table 4). The AIC and BIC statistics suggested that the generalized gamma model would be a better fit for the data than the Weibull model, and thus the generalized gamma model was selected as the model of choice. AFs of >1.0 indicated that switchers experienced extended survival compared with nonswitchers in both the enzalutamide and placebo groups. The generalized gamma model suggested that this benefit was slightly greater in the placebo group.
Adjusted HRs for OS for the two-stage method are shown in Table 4, together with the median times to event. Kaplan–Meier curves (Fig. 2) showed that a substantial amount of information was lost with recensoring of the data, creating the potential for considerable bias under the assumption that the treatment effect changed over time. Thus, the preferred two-stage approach was considered to be one that used a generalized gamma model and did not incorporate recensoring. This yielded an adjusted HR for OS of 0.66 (95% CI 0.57–0.81) for enzalutamide versus placebo (Table 5).
More detailed information relating to these results, including information pertaining to sensitivity analyses, is provided in ESM 1.
3.2.3 IPCW Method
Adjusting for treatment switching of 14.8% and 21.3% in enzalutamide and placebo patients, respectively, the IPCW resulted in a HR for OS of 0.63 (95% CI 0.52–0.75) versus placebo (Table 4). Weighted Kaplan–Meier curves are shown in Fig. 3. Stabilized weights were utilized and, therefore, the IPCW HR was adjusted for baseline covariates. However, the weighted Kaplan–Meier curves shown in Fig. 3 were not adjusted for baseline covariates.
An exploration of the distribution of the stabilized weights was performed (see ESM 7), which showed that for both treatment arms and all follow-up intervals the mean was very close to 1. For enzalutamide, the smallest and largest weight was 0.91 and 2.21, respectively, which is comparable to 0.96 and 3.25, respectively, for placebo. More detailed information, including data pertaining to sensitivity analyses, is provided in ESM 1, 7 and 8.
3.3 Updated OS Analysis
A subsequent analysis was performed, in line with the FDA request to analyze the OS endpoint with a minimum of the original protocol-defined number of deaths (756), at a data cutoff of June 1, 2014. At this time point, 784 deaths had occurred, with 99.9% (844/845) of placebo-treated patients and 73.3% (639/872) of enzalutamide-treated patients having discontinued treatment. (As previously mentioned, one patient in each treatment group had not received study medication.) There were 416 switchers, representing 19.7% (172/872) and 28.9% (244/845) of patients in the enzalutamide and placebo arms, respectively. The HR for OS for enzalutamide versus placebo was 0.77 (95% CI 0.67–0.88) in the ITT analysis, compared with 0.74 (95% CI 0.64–0.86) in the two-stage method (generalized gamma without recensoring) and 0.66 (95% CI 0.57–0.77) in the IPCW analysis.
4 Discussion
The original results from the PREVAIL study demonstrated an OS benefit with enzalutamide for the treatment of mCRPC in chemotherapy-naïve patients [10]. That analysis, performed after 540 reported deaths, showed evidence of a significant 29% reduction in the risk of death for patients originally randomized to enzalutamide versus placebo (HR 0.71; 95% CI 0.60–0.84) [10]. However, for an analysis of the treatment benefit that would be expected if enzalutamide was to be incorporated into the treatment pathway in the UK, it is necessary to adjust for any treatments received during the PREVAIL trial that are outside the standard UK treatment pathway (other than the enzalutamide received in the experimental arm of the study). The original results from the PREVAIL study used standard ITT methodology, which may inaccurately estimate the true OS benefit associated with an original investigational product when switching to another active treatment subsequently occurs [2], as is frequently the case in oncology trials if cancer progresses. Consequently, in an attempt to provide a better estimate of the effect of enzalutamide on OS in a UK-specific context, adjustments for switching to active postprogression antineoplastic therapies that were not part of the standard UK treatment pathway were made using the observational-based two-stage [5, 6] and IPCW [6] methods. Both methods resulted in a numerical reduction in the HR compared with the unadjusted analysis, producing values of 0.66 (95% CI 0.57–0.81) and 0.63 (95% CI 0.52–0.75), respectively, suggestive of a slightly larger effect of enzalutamide on OS compared with the interim analysis (HR 0.71; 95% CI 0.60–0.84) [10]. Thus, by adjusting for switching to nonstandard antineoplastic therapies in 14.8% (129/872) of enzalutamide- and 21.3% (180/845) of placebo-treated patients, enzalutamide was shown to be associated with an approximately 34% reduction in mortality risk with the two-stage method and a decrease of approximately 37% using the IPCW analysis.
A subsequent OS analysis, performed at a data cutoff associated with 784 deaths, also showed that the adjustment methods produced numerical reductions in the HR compared with the ITT analysis for OS for enzalutamide versus placebo at this time point. Given the potential importance of reductions in the HR for clinical and reimbursement decision-making, it is appropriate to consider our methodological choices in more detail.
After discontinuation of the study drug, patients in both arms of the PREVAIL trial switched to several different treatments. Randomization-based methodologies, such as the RPSFTM or IPE algorithm, were not considered to be suitable for adjustment, as they are typically unable to adequately account for switching when patients may receive a variety of poststudy active therapies; these methods are appropriate when switching is directly from the comparator treatment to the randomized treatment [2]. Although a multiparameter RPSFTM may be considered to model more complex switching scenarios, in practice, such methodology has been shown to be unhelpful [16–20].
Having assessed the pivotal assumptions underlying the available adjustment methods, and taking into account the characteristics of the PREVAIL study [10] and treatment switching mechanism, we regarded the observational-based two-stage method as applicable to the PREVAIL data. However, this method required an appropriate secondary baseline and, with the PREVAIL study not specifying any particular point (time or event, such as disease progression) at which switching could occur, and no actual switching date available for nonswitchers, the treatment discontinuation date was used. This is a potential weakness of the analysis, as it relies on the assumption that the secondary baseline represents a similar disease-related time point for all patients. This is more obviously the case when the secondary baseline is the time of disease progression than when it is the time of treatment discontinuation. Nevertheless, with patients originally randomized to both enzalutamide and placebo having switched treatment, and the primary reason for discontinuing study drug being disease progression, the treatment discontinuation date may be considered to represent an adequate disease-related time point for an appropriate secondary baseline. A large number of variables potentially prognostic for survival and capable of enabling accurate adjustment for differences between switchers and nonswitchers had been assessed in the study up to this time point and were available for use as covariates in the two-stage analysis. Therefore, it was considered appropriate to assume that the no unmeasured confounders assumption was approximately true.
The two-stage adjustment method can be applied in a number of different ways, such as (1) with and without recensoring and (2) using different accelerated failure time models to estimate the effect of switching. Our preferred approach was to use the generalized gamma model without recensoring. The practice of recensoring is generally recommended for approaches that estimate counterfactual survival times as it reduces bias by breaking the dependence between censoring time and treatment [16]. However, recensoring was considered inappropriate in the current study as this led to the loss of a substantial amount of long-term information, creating the potential for considerable bias if the treatment effect changes over time. In the Kaplan–Meier analyses (Fig. 2b), the enzalutamide curve showed little change after adjustment, whereas a slight worsening of survival was apparent for the placebo group. The magnitude of such changes was in line with expectations, given the low proportions of switchers (14.8 and 21.3% of patients in the enzalutamide and placebo groups, respectively) and the fact that adjustment has been made for a treatment effect likely to be relatively small—although the AF was >1.0, indicating a treatment benefit associated with switching, it was neither substantially greater than this nor statistically significant.
The IPCW method was also considered to be suitable for this post-hoc analysis of PREVAIL data because a large number of covariates likely to be prognostic for survival and with the potential to predict an investigator’s decision to switch a patient’s treatment were collected in the trial. Therefore, we could reasonably assume that the no unmeasured confounders assumption was plausible. This adjustment method involves censoring when patients switch to other treatments and may thus be prone to bias if this proportion is very high. However, this was not the case in PREVAIL where there were relatively low proportions of switchers. Therefore, the IPCW, acknowledged as a valid option to correct for switching bias [21–23], was considered to be appropriate. When applying the IPCW method it is important to explore the distributions of the weights. In some instances a poor performing covariate will cause many extreme weights, and extreme weights can cause the resultant analysis to be prone to error. An exploration of the distribution of stabilized weights showed that the mean was very close to 1.0 for both treatments and all follow-up intervals, with no extreme weights observed.
An important limitation associated with the IPCW as applied to PREVAIL was that no covariate data were collected after treatment discontinuation in the trial. Therefore, time-dependent confounding could occur between the time of treatment discontinuation and the time of treatment switch. This negates one of the important theoretical advantages that the IPCW holds over the two-stage method—in theory, the IPCW adjusts for any patient characteristic differences that occur between the time point of the secondary baseline and the time of treatment switch, whereas the two-stage method does not. However, given the data collected in PREVAIL, both adjustment methods were prone to this confounding. In relation to this, it is relevant to note that the interval between the secondary baseline and the time of switch was not substantial and was similar between the enzalutamide and placebo arms [mean 1.98 (median 1.25) vs. mean 2.28 (median 1.28) months, respectively] and could have been a consequence of logistical delays in the administration of new antineoplastic treatment.
Another limitation, applicable to both the two-stage and IPCW methods, was reliance on a key assumption of there being no unmeasured confounders. Although this assumption could not be tested in practice, the methods were likely to have performed acceptably if it was approximately true, such that any important independent predictive variables were taken into account [2]. With the list of covariates being based on clinical expert opinion and prior knowledge from the literature, and a considerable volume of data having being collected in PREVAIL, the assumption of no unmeasured confounders may be regarded as reasonable in this instance.
The two-stage method predicted that the switching effect (in terms of improved survival after switching to nonstandard active treatments) was greater in the placebo group of switchers than in the enzalutamide group of switchers. It is possible that this could have been a consequence of cross-resistance [24, 25] affecting enzalutamide-treated patients switching to abiraterone, which was the most common of the nonstandard subsequent first antineoplastic therapies. Further information on potential cross-resistances would be valuable.
5 Conclusion
Overall, in line with the original analysis from the PREVAIL study [10], our adjusted analyses demonstrated the OS benefit of enzalutamide versus placebo for men with chemotherapy-naïve mCRPC. Both adjustment methods produced similar results, which increases confidence that they have performed appropriately and produced credible results, with HR suggestive of a slightly higher treatment effect than apparent with the original ITT analysis. This result is important for interpreting the results of the trial both from a clinical and a cost-effectiveness point of view. Although these analyses have been undertaken from a UK treatment pathway perspective, such adjustment methods may also be relevant for economic analyses in other countries, being tailored to take into account the treatment pathways in the countries under consideration.
References
Latimer NR. Treatment switching in oncology trials and the acceptability of adjustment methods. Expert Rev Pharmacoecon Outcomes Res. 2015;15(4):561–4.
Latimer NR, Abrams KR, Lambert PC, et al. Adjusting survival time estimates to account for treatment switching in randomized controlled trials--an economic evaluation context: methods, limitations, and recommendations. Med Decis Mak. 2014;34(3):387–402.
Robins JM, Tsiatis AA. Correcting for noncompliance in randomized trials using rank preserving structural failure time models. Commun Stat Theory Methods. 1991;20(8):2609–31.
Branson M, Whitehead J. Estimating a treatment effect in survival studies in which patients switch treatment. Stat Med. 2002;21(17):2449–63.
Latimer NR, Abrams K, Lambert P, et al. Adjusting for treatment switching in randomised controlled trials—A simulation study and a simplified two-stage method. Stat Methods Med Res. 2014. doi: 10.1177/0962280214557578
Latimer NR, Abrams KR, Lambert PC, et al. Assessing methods for dealing with treatment switching in clinical trials: A follow-up simulation study. Stat Methods Med Res. 2016. doi: 10.1177/0962280216642264
Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–88.
Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90.
Scher HI, Fizazi K, Saad F, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.
Beer TM, Armstrong AJ, Rathkopf DE, et al. PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33.
Astellas Pharma Ltd. Xtandi® (enzalutamide) Summary of Product Characteristics. 2015. Available at: https://www.medicines.org.uk/emc/medicine/27912/SPC/Xtandi+40mg+soft+capsules/. Accessed 3 June 2016.
Astellas Pharma US, Inc. Xtandi® (enzalutamide) Prescribing information. 2015. Available at: http://www.astellas.us/docs/us/12A005-ENZ-WPI.pdf?v=1. Accessed 3 June 2016.
National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal. 2013. Available at: http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf. Accessed Jun 3, 2016.
NICE. Clinical guideline 175. Prostate cancer: diagnosis and treatment. 2014. Available at: http://www.nice.org.uk/guidance/cg175. Accessed 3 June 2016.
Latimer NR, Abrams KR. NICE DS technical support document 16: Adjusting survival time estimates in the presence of treatment switching. Report by the Decision Support Unit. 2014. Available at: http://www.nicedsu.org.uk/TSD16_Treatment_Switching.pdf. Accessed 3 June 2016.
White IR, Babiker AG, Walker S, et al. Randomization-based methods for correcting for treatment changes: examples from the Concorde trial. Stat Med. 1999;18(19):2617–34.
Beer TM, Armstrong AJ, Sternberg CN, et al. Enzalutamide (ENZA) in men with chemotherapy-Naïve metastatic castration-resistant prostate cancer (mCRPC): Final analysis of the phase 3 PREVAIL study. J Clin Oncol. 2015;33[15 Suppl]:abstr 5036.
Robins J, Greenland S. Adjusting for differential rates of prophylaxis therapy for PCP in high- versus low-dose AZT treatment in an AIDS randomized trial. J Am Stat Assoc. 1994;89(427):737–49.
Yamaguchi T, Ohashi Y. Adjusting for differential proportions of second-line treatment in cancer clinical trials. Part I: Structural nested models and marginal structural models to test and estimate treatment arm effects. Stat Med. 2004;23(13):1991–2003.
Yamaguchi T, Ohashi Y. Adjusting for differential proportions of second-line treatment in cancer clinical trials. Part II: an application in a clinical trial of unresectable non-small-cell lung cancer. Stat Med. 2004;23(13):2005–22.
NICE. Final appraisal determination—Imatinib for the adjuvant treatment of gastrointestinal stromal tumours (review of NICE technology appraisal guidance 196). 2014. Available at: https://www.nice.org.uk/guidance/ta326/documents/gastrointestinal-stromal-tumours-imatinib-adjuvant-rev-ta196-id696-final-appraisal-determination-document2. Accessed 3 June 2016.
NICE. Final appraisal determination—Crizotinib for previously treated non-small-cell lung cancer associated with an anaplastic lymphoma kinase fusion gene. 2013. Available at: https://www.nice.org.uk/guidance/ta296/documents/lung-cancer-nonsmallcell-anaplastic-lymphoma-kinase-fusion-gene-previously-treated-crizotinib-final-appraisal-determination3. Accessed Jun 3, 2016.
NICE. Technology appraisal guidance [TA219]: Everolimus for the second-line treatment of advanced renal cell carcinoma. 2011. Available at: http://guidance.nice.org.uk/TA219. Accessed 3 June 2016.
Omlin A, Pezaro C, Gillessen Sommer S. Sequential use of novel therapeutics in advanced prostate cancer following docetaxel chemotherapy. Ther Adv Urol. 2014;6(1):3–14.
Sartor O, Gillessen S. Treatment sequencing in metastatic castrate-resistant prostate cancer. Asian J Androl. 2014;16(3):426–31.
Acknowledgments
Medical writing assistance, provided by Thomas Lavelle and Andy Lockley of Bioscript Science, and editorial assistance, provided by Shannon Davis of Ashfield Healthcare Communications, were funded by the study sponsors.
Author’s Contributions
All authors contributed to the concept and design of the study. Shevani Naidoo, De Phung, and Stefan Holmstrom contributed to the collection and/or assembly of data. Konstantina Skaltsa, Cristina Ivanescu, and Nicholas R. Latimer contributed to the data analysis and interpretation. All authors contributed to the writing of the manuscript. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was funded by Astellas Pharma, Inc., and Medivation, Inc., the co-developers of enzalutamide. Medivation was acquired by Pfizer, Inc., in September 2016.
Conflict of Interest
Konstantina Skaltsa and Cristina Ivanescu are employees of Quintiles, which received consulting fees from Astellas for the analysis and interpretation of data from the PREVAIL trial. Nicholas R. Latimer has acted in a consultancy or advisory role for GlaxoSmithKline, Pfizer, Sanofi, Astellas (including for the work presented in this manuscript), Amgen, AstraZeneca, Janssen, Roche, and Bayer and has received research funding from Novartis, GlaxoSmithKline, and the Pharmacology Oncology Initiative. De Phung, Stefan Holmstrom, and Shevani Naidoo are employees of Astellas.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Online Resource 1
(DOCX 39 kb)
Online Resource 2
(DOCX 30 kb)
Online Resource 3
(DOCX 30 kb)
Online Resource 4
(DOCX 31 kb)
Online Resource 5
(DOCX 32 kb)
Online Resource 6
(DOCX 29 kb)
Online Resource 7
(DOCX 28 kb)
Online Resource 8
(DOCX 27 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Skaltsa, K., Ivanescu, C., Naidoo, S. et al. Adjusting Overall Survival Estimates after Treatment Switching: a Case Study in Metastatic Castration-Resistant Prostate Cancer. Targ Oncol 12, 111–121 (2017). https://doi.org/10.1007/s11523-016-0472-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-016-0472-3