Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease with a survival rate of 4–6 months from diagnosis. PDAC is the fourth leading cause of cancer-related death in the Western world, with a mortality rate of 10 cases per 100,000 population. Chemotherapy constitutes only a palliative strategy, with limited effects on life expectancy.
Aims
To investigate the biological response of PDAC to mitogen-activated protein kinase (MAPK) and NF-kappaB (NF-kB) inhibitors and the role of autophagy in the modulation of these signaling pathways in order to address the challenge of developing improved medical protocols for patients with PDAC.
Methods
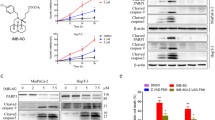
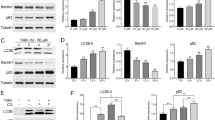
Two ATCC cell lines, MIAPaCa-2 and PANC-1, were used as PDAC models. Cells were exposed to inhibitors of MAPK or NF-kB survival pathways alone or after autophagy inhibition. Several aspects were analyzed, as follows: cell proliferation, by [3H]TdR incorporation; cell death, by TUNEL assay, regulation of autophagy by LC3-II expression level and modulation of pro-and anti-apoptotic proteins by Western blot.
Results
We demonstrated that the inhibition of the MAPK and NF-kB survival pathways with U0126 and caffeic acid phenethyl ester (CAPE), respectively, produced strong inhibition of pancreatic tumor cell growth without inducing apoptotic death. Interestingly, U0126 and CAPE induced apoptosis after autophagy inhibition in a caspase-dependent manner in MIA PaCa-2 cells and in a caspase-independent manner in PANC-1 cells.
Conclusions
Here we present evidence that allows us to consider a combined therapy regimen comprising an autophagy inhibitor and a MAPK or NF-kB pathway inhibitor as a possible treatment strategy for pancreatic cancer.






Similar content being viewed by others
References
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362(17):1605–17
Hidalgo M (2012) New insights into pancreatic cancer biology. Ann Oncol 23(Suppl 10):135–8
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J Clin 62:10–29
O'Reilly KE, Rojo F, She QB et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–8
Garcia MG, Alaniz LD, Cordo Russo RI et al (2009) PI3K/Akt inhibition modulates multidrug resistance and activates NFkB in murine Lymphoma cell lines. Leuk Res 33:288–96
Cox AD, Der CJ (1997) Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim Biophys Acta 1333(1):F51–71
Muerkoster S, Arlt A, Sipos B et al (2005) Increased expression of the E3-ubiquitin ligase receptor subunit betaTRCP1 relates to constitutive nuclear factor-kappaB activation and chemoresistance in pancreatic carcinoma cells. Cancer Res 65(4):1316–24
Aksamitiene E, Kiyatkin A, Kholodenko BN (2012) Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans 40:139–46
De Luca A, Maiello MR, D'Alessio A et al (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 16:S17–27
Shimizu T, Tolcher AW, Papadopoulos KP et al (2012) The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res 18:2316–25
Wang LH (2014) LiY, Yang SN, et al. Gambogic acid synergistically potentiates cisplatin-induced apoptosis in non-small-cell lung cancer through suppressing NF-κB and MAPK/HO-1 signalling. Br J Cancer 110(2):34–52
Sylvester RJ, van der Meijden AP, Oosterlinck W et al (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49:466–75
Vaux DL, Silke J (2003) Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun 304:499–504
Wei Y, Fan T, Yu M (2008) Inhibitor of apoptosis proteins and apoptosis. Acta Biochim Biophys Sin (Shanghai) 40:278–88
Srinivasula SM, Ashwell JD (2008) IAPs: what's in a name? Mol Cell 30:123–35
Dubrez-Daloz L, Dupoux A, Cartier J (2008) IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle 7:1036–46
LaCasse EC, Mahoney DJ, Cheung HH et al (2008) IAP-targeted therapies for cancer. Oncogene 27:6252–75
Vucic D, Fairbrother WJ (2007) The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res 13:5995–6000
Reggiori F, Klionsky DJ (2002) Autophagy in the eukaryotic cell. Eukaryot Cell 1(1):11–21
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–5
Kirkegaard K, Taylor MP, Jackson WT (2004) Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2(4):301–14
Ogawa M, Yoshimori T, Suzuki T et al (2005) Escape of intracellular Shigella from autophagy. Science 307(5710):727–31
Hara T, Nakamura K, Matsui M et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441(7095):885–9
Komatsu M, Waguri S, Chiba T et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–4
Liang XH, Jackson S, Seaman M et al (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402(6762):672–6
Liang XH, Yu J, Brown K et al (2001) Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer Res 61(8):3443–9
Ravikumar B, Berger Z, Vacher C et al (2006) Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet 15(7):1209–16
Papademetrio DL, Cavaliere V, Simunovich T et al (2014) Interplay between autophagy and apoptosis in pancreatic tumors in response to gemcitabine. Target Oncol 9(2):123–34
Kabeya Y, Mizushima N, Ueno T et al (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19(21):5720–8
Rubinsztein DC, Cuervo AM, Ravikumar B et al (2009) In search of an "autophagomometer". Autophagy 5(5):585–9
Conroy T, Desseigne F, Ychou M et al (2001) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–25
Hill R, Rabb M, Madureira PA et al (2013) Gemcitabine-mediated tumour regression and p53-dependent gene expression: implications for colon and pancreatic cancer therapy. Cell Death Dis 4:e791
Jones S, Zhang X, Parsons DW et al (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–6
Biankin AV, Waddell N, Kassahn KS et al (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491:399–405
Baines AT, Xu D, Der CJ (2011) Inhibition of Ras for cancer treatment: the search continues. Future Med Chem 3:1787–808
Chen Z, Cheng K, Walton Z et al (2012) A murine lung cancer coclinical trial identifies genetic modifiers of therapeutic response. Nature 483(7391):613–7
Jänne PA, Shaw AT, Pereira JR et al (2013) Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 14:38–47
McCubrey JA, Steelman LS, Chappell WH et al (2012) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget 3:1068–111
Fujioka S, Sclabas GM, Schmidt C et al (2003) Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res 9:346–54
Hu L, Shi Y, Hsu JH et al (2003) Downstream effectors of oncogenic ras in multiple myeloma cells. Blood 101:3126–35
Mayo MW, Wang CY, Cogswell PC et al (1997) Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278:1812–5
Li L, Aggarwal BB, Shishodia S et al (2004) Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 101:2351–62
Aggarwal BB (2004) Nuclear factor-kappaB: the enemy within. Cancer Cell 6:203–8
Yamamoto Y, Gaynor RB (2001) Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr Mol Med 1:287–96
Aggarwal BB, Takada Y, Shishodia S et al (2004) Nuclear transcription factor NF-kappa B: role in biology and medicine. Indian J Exp Biol 42:341–53
Karin M, Cao Y, Greten FR et al (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2:301–10
Garg A, Aggarwal BB (2002) Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia 16:1053–68
Lin Y, Shi R, Wang X et al (2008) Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 8:634–46
Cai X, Lu W, Yang Y et al (2013) Digitoflavone inhibits IκBα kinase and enhances apoptosis induced by TNFα through downregulation of expression of nuclear factor κB-regulated gene products in human pancreatic cancer cells. PLoS One 8(10):e77126
Cavaliere V, Papademetrio DL, Lorenzetti M et al (2009) Caffeic Acid Phenylethyl Ester and MG-132 have apoptotic and antiproliferative effects on Leukemic cells but not on normal mononuclear cells. Transl Oncol 2(1):46–58
Velculescu VE (1999) Essay: Amersham Pharmacia Biotech & Science prize. Tantalizing transcriptomes SAGE and its use in global gene expression analysis. Science 286(5444):1491–2
Altieri DC (2008) Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8(1):61–70
Sarela AI, Macadam RC, Farmery SM et al (2000) Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut 46(5):645–50
Monzo M, Rosell R, Felip E et al (1999) A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol 17(7):2100–4
Shariat SF, Lotan Y, Saboorian H et al (2004) Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer 100(4):751–7
Tanaka K, Iwamoto S, Gon G et al (2000) Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Can 6(1):127–34
Jourdan M, Reme T, Goldschmidt H et al (2009) Gene expression of anti- and pro-apoptotic proteins in malignant and normal plasma cells. Br J Haematol 145(1):45–58
Cheng SM, Chang YC, Liu CY et al (2015) YM155 down-regulates survivin and XIAP, modulates autophagy and induces autophagy-dependent DNA damage in breast cancer cells. Br J Pharmacol 172(1):214–34
Wang J, Whiteman MW, Lian H et al (2009) A Non-canonical MEK/ERK Signaling Pathway Regulates Autophagy via Regulating Beclin 1. J Biol Chem 284(32):21412–24
Pattingre S, Bauvy C, Codogno PZ (2003) Amino acids interfere with the ERK1⁄ 2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J Biol Chem 278:16667–74
Ellington AA, Berhow MA, Singletary KW (2006) Inhibition of Akt signaling and enhanced ERK1⁄ 2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis 27:298–306
Copetti T, Demarchi F, Schneider C (2009) p65/RelA binds and activates the beclin 1 promoter. Autophagy 5(6):858–9
Vadlamudi RK, Shin J (1998) Genomic structure and promoter analysis of the p62 gene encoding a nonproteasomal multiubiquitin chain binding protein. FEBS Lett 435:138–42
David A (2014) An autophagic switch in the response of tumor cells to radiation and chemotherapy. Biochem Pharmacol 90:208–11
Yang S, Wang X, Contino G et al (2011) Pancreatic cancers require autophagy for tumor growth. Genes Dev 25(7):717–29
Acknowledgments
The authors thank Dr. Daniela Ureta (Servicio de Citometría de flujo, Departamento de Microbiología, Inmunología y Biotecnología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Argentina) for technical assistance and Martín Levermann for language assistance during the edition of the manuscript.
Compliance with Ethical Standards
ᅟ
Funding
This study was funded by Universidad de Buenos Aires and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Conflict of Interest
The authors (DLP, SLL, TS, SC, CYM, VC, EA) declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Papademetrio, D.L., Lompardía, S.L., Simunovich, T. et al. Inhibition of Survival Pathways MAPK and NF-kB Triggers Apoptosis in Pancreatic Ductal Adenocarcinoma Cells via Suppression of Autophagy. Targ Oncol 11, 183–195 (2016). https://doi.org/10.1007/s11523-015-0388-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-015-0388-3