Abstract
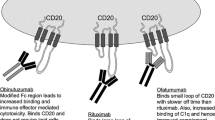
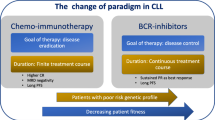
The introduction of targeted agents such as the monoclonal antibodies rituximab (anti-CD20) and alemtuzumab (anti-CD52) has brought about a remarkable change in the therapy of chronic lymphocytic leukemia (CLL). Although it is unclear whether overall survival has been improved, the incorporation of these monoclonal antibodies into chemoimmunotherapy regimens has dramatically improved complete response rates and progression-free survival in patients with both newly-diagnosed and relapsed CLL. The success of rituximab and alemtuzumab has spurred the development of other monoclonal antibodies targeting distinct proteins and epitopes on the surface of CLL cells and an exciting array of novel immunotherapeutics. The judicious use of these agents provides the opportunity to develop risk-adapted therapeutic strategies to optimize responses and quality of life in patients with CLL.
Similar content being viewed by others
References
Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Brenner H, Gondos A, Pulte D (2008) Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood 111:4916–4921
Cheson BD, Bennett JM, Grever M et al (1996) National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 87:4990–7
Lozanski G, Heerema NA, Flinn IW et al (2004) Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 103:3278–3281
Dohner H, Fischer K, Bentz M et al (1995) p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood 85:1580–1589
Dohner H, Stilgenbauer S, Benner A et al (2000) Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 343:1910–1916
Reff ME, Carner K, Chambers KS et al (1994) Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83:435–445
Beum PV, Lindorfer MA, Beurskens F et al (2008) Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol 181:822–832
Golay J, Lazzari M, Facchinetti V et al (2001) CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood 98:3383–3389
Golay J, Zaffaroni L, Vaccari T et al (2000) Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 95:3900–3908
Treon SP, Mitsiades C, Mitsiades N et al (2001) Tumor cell expression of CD59 is associated with resistance to CD20 serotherapy in patients with B-cell malignancies. J Immunother 24:263–271
Pedersen IM, Buhl AM, Klausen P, Geisler CH, Jurlander J (2002) The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood 99:1314–1319
Alas S, Emmanouilides C, Bonavida B (2001) Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin’s lymphoma to apoptosis. Clin Cancer Res 7:709–723
Maloney DG, Liles TM, Czerwinski DK et al (1994) Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 84:2457–2466
Maloney DG, Grillo-Lopez AJ, Bodkin DJ et al (1997) IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol 15:3266–3274
Maloney DG, Grillo-Lopez AJ, White CA et al (1997) IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 90:2188–2195
McLaughlin P, Grillo-Lopez AJ, Link BK et al (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833
Grillo-Lopez AJ (2000) Rituximab: an insider’s historical perspective. Semin Oncol 27:9–16
McLaughlin P, White CA, Grillo-Lopez AJ, Maloney DG (1998) Clinical status and optimal use of rituximab for B-cell lymphomas. Oncology (Williston Park) 12:1763–1769 discussion 1769–1770, 1775–1777
Nguyen DT, Amess JA, Doughty H, Hendry L, Diamond LW (1999) IDEC-C2B8 anti-CD20 (rituximab) immunotherapy in patients with low-grade non-Hodgkin’s lymphoma and lymphoproliferative disorders: evaluation of response on 48 patients. Eur J Haematol 62:76–82
Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A (1999) Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 94:2217–2224
Ladetto M, Bergui L, Ricca I, Campana S, Pileri A, Tarella C (2000) Rituximab anti-CD20 monoclonal antibody induces marked but transient reductions of peripheral blood lymphocytes in chronic lymphocytic leukaemia patients. Med Oncol 17:203–210
Huhn D, von Schilling C, Wilhelm M et al (2001) Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood 98:1326–1331
Itala M, Geisler CH, Kimby E et al (2002) Standard-dose anti-CD20 antibody rituximab has efficacy in chronic lymphocytic leukaemia: results from a Nordic multicentre study. Eur J Haematol 69:129–134
Hainsworth JD, Litchy S, Barton JH et al (2003) Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 21:1746–1751
Thomas D, O’Brien S, Giles FJ et al (2001) Single agent rituxan in early stage chronic lymphocytic leukemia (CLL). Blood 98:364
O’Brien SM, Kantarjian H, Thomas DA et al (2001) Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 19:2165–2170
Byrd JC, Murphy T, Howard RS et al (2001) Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 19:2153–2164
Byrd JC, Peterson BL, Morrison VA et al (2003) Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 101:6–14
Wierda W, O’Brien S, Wen S et al (2005) Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 23:4070–4078
Keating MJ, O’Brien S, Albitar M et al (2005) Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 23:4079–4088
Tam CS, O’Brien S, Wierda W et al (2008) Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 112:975–980
Lamanna N, Kalaycio M, Maslak P et al (2006) Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol 24:1575–1781
Kay NE, Geyer SM, Call TG et al (2007) Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 109:405–411
Hale G, Bright S, Chumbley G et al (1983) Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood 62:873–882
Hale G, Swirsky D, Waldmann H, Chan LC (1985) Reactivity of rat monoclonal antibody CAMPATH-1 with human leukaemia cells and its possible application for autologous bone marrow transplantation. Br J Haematol 60:41–48
Xia MQ, Tone M, Packman L, Hale G, Waldmann H (1991) Characterization of the CAMPATH-1 (CDw52) antigen: biochemical analysis and cDNA cloning reveal an unusually small peptide backbone. Eur J Immunol 21:1677–1684
Gilleece MH, Dexter TM (1993) Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood 82:807–812
Rowan W, Tite J, Topley P, Brett SJ (1998) Cross-linking of the CAMPATH-1 antigen (CD52) mediates growth inhibition in human B- and T-lymphoma cell lines, and subsequent emergence of CD52-deficient cells. Immunology 95:427–436
Bowen AL, Zomas A, Emmett E, Matutes E, Dyer MJ, Catovsky D (1997) Subcutaneous CAMPATH-1H in fludarabine-resistant/relapsed chronic lymphocytic and B-prolymphocytic leukaemia. Br J Haematol 96:617–619
Keating MJ, Flinn I, Jain V et al (2002) Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 99:3554–3561
Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H (1996) Humanized CD52 monoclonal antibody Campath-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol 93:151–153
Osterborg A, Dyer MJ, Bunjes D et al (1997) Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol 15:1567–1574
Rai KR, Freter CE, Mercier RJ et al (2002) Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol 20:3891–3897
Stilgenbauer S WD, Krober A, et al. (2004) Subcutaneous campath-1H (alemtuzumab) in fludarabine-refractory cll: interim analysis of the CLL2H study of the German CLL Study Group (GCLLSG). Blood 104:(abstract 478)
Montillo M TA, Rossi V, et al. (2004) Alemtuzumab as consolidation after a response to fludarabine is effective to purge residual disease in patients with chronic lymphocytic leukemia. Blood 104:(abstract 479)
Moreton P, Kennedy B, Lucas G et al (2005) Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol 23:2971–2979
Stilgenbauer S, Dohner H (2002) Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy. N Engl J Med 347:452–453
Hillmen P, Skotnicki AB, Robak T et al (2007) Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 25:5616–5623
Elter T, Borchmann P, Schulz H et al (2005) Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: results of a phase II trial. J Clin Oncol 23:7024–7031
Wierda W, O’Brien S, Faderl S et al (2006) Combined cyclophosphamide, fludarabine, alemtuzumab, and rituximab (CFAR), an active regimen for heavily treated patients with CLL. Blood 108:14a
O’Brien SM, Kantarjian HM, Thomas DA et al (2003) Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer 98:2657–2663
Wendtner CM, Ritgen M, Schweighofer CD et al (2004) Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission—experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG). Leukemia 18:1093–1101
Rai K, Byrd, JC, Peterson, B, et al. (2003) Subcutaneous alemtuzumab following fludarabine for previously untreated patients with chronic lymphocytic leukemia (CLL): CALGB study 1990. Blood 102:(abstract 2506).
Montillo M, Tedeschi A, Miqueleiz S et al (2006) Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol 24:2337–2342
Lin TS, Donohue KA, Lucas MS, et al. (2007) Consolidation therapy with subcutaneous (SC) alemtuzumab results in severe infectious toxicity in previously untreated CLL patients who achieve a complete response (CR) after fludarabine and rituximab (FR) induction therapy: interim safety analysis of the CALGB study 10101. Blood 110:(abstract 755)
Faderl S, Thomas DA, O’Brien S et al (2003) Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood 101:3413–3415
Zent CS, Call TG, Shanafelt TD et al (2008) Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer 113:2110–2118
Pathan NZA, Wynne D et al (2003) Lumiliximab (IDEC-152) an anti-CD23 antibody, induces apoptosis in vitro and in vivo in CLL cells. Blood 102:438a
Mangiola M, Welsh K, Kitada S et al (2004) Anti-CD20 antibody rituximab and anti-CD23 antibody IDEC-152 induce apoptosis of malignant B-cells in combination with chemical antagonists of XIAP. Blood 11:393a
Pathan N, Hopkins M, Saven A, et al. (2001) Induction of apoptosis by IDEC-152 (anti-CD23) in chronic lymphocytic leukemia. Leuk Lymphoma 42
Byrd JC, O’Brien S, Flinn IW et al (2007) Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res 13:4448–4455
Byrd J, Castro J, O’Brien S et al (2006) Comparison of results from a phase 1/2 study of lumiliximab (anti-CD23) in combination with FCR for patients with relapsed CLL with published FCR results. Blood 11:14a
Teeling JL, Mackus WJ, Wiegman LJ et al (2006) The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 177:362–371
Teeling JL, French RR, Cragg MS et al (2004) Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104:1793–1800
Coiffier B, Tilly H, Pedersen LM, et al. (2006) Significant correlation between survival endpoints and exposure to ofatumumab (HuMax-CD20) in chronic lymphocytic leukemia. Blood 11:(abstract 2842)
Bowles JA, Wang SY, Link BK et al (2006) Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood 108:2648–2654
Umana P, Ekkehard M, Bruenker P, et al. (2006) Novel 3rd generation humanized type II CD20 antibody with glycoengineered Fc and modified elbow hinge for enhanced ADCC and superior apoptosis induction. Blood 11:(abstract 229)
Stein R, Qu Z, Chen S et al (2004) Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and Its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin’s lymphoma. Clin Cancer Res 10:2868–2878
Morshhauser F, Leonard JP, Coiffier B, et al. (2006) Phase I/II results of a second-generation humanized anti-CD20 antibody, IMMU-106 (hA20), in NHL. Proc Am Soc Clin Oncol 24:(abstract 7530)
Leonard JP, Coleman M, Ketas JC et al (2003) Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J Clin Oncol 21:3051–3059
Leonard JP, Coleman M, Ketas J et al (2005) Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol 23:5044–5051
Kostelny SA, Link BK, Tso JY et al (2001) Humanization and characterization of the anti-HLA-DR antibody 1D10. Int J Cancer 93:556–565
Qu Z, Goldenberg DM, Cardillo TM, Shi V, Hansen HJ, Chang CH (2008) Bispecific anti-CD20/22 antibodies inhibit B-cell lymphoma proliferation by a unique mechanism of action. Blood 111:2211–2219
Bargou R, Leo E, Zugmaier G et al (2008) Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321:974–977
Conflict of interest statement
No funds were received in support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quintás-Cardama, A., O’Brien, S. Targeted therapy for chronic lymphocytic leukemia. Targ Oncol 4, 11–21 (2009). https://doi.org/10.1007/s11523-008-0099-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-008-0099-0