Abstract
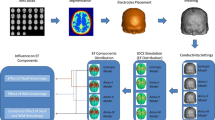
The aim was to quantify the influence of heterogeneous isotropic and heterogeneous anisotropic tissue on the spatial distribution of the electric field during deep brain stimulation (DBS). Three finite element tissue models were created of one patient treated with DBS. Tissue conductivity was modelled as (I) homogeneous isotropic, (II) heterogeneous isotropic based on MRI, and (III) heterogeneous anisotropic based on diffusion tensor MRI. Modelled DBS electrodes were positioned in the subthalamic area, the pallidum, and the internal capsule in each tissue model. Electric fields generated during DBS were simulated for each model and target-combination and visualized with isolevels at 0.20 (inner), and 0.05 V mm−1 (outer). Statistical and vector analysis was used for evaluation of the distribution of the electric field. Heterogeneous isotropic tissue altered the spatial distribution of the electric field by up to 4% at inner, and up to 10% at outer isolevel. Heterogeneous anisotropic tissue influenced the distribution of the electric field by up to 18 and 15% at each isolevel, respectively. The influence of heterogeneous and anisotropic tissue on the electric field may be clinically relevant in anatomic regions that are functionally subdivided and surrounded by multiple fibres of passage.





Similar content being viewed by others
References
Pahwa R, Wilkinson SB, Overman J, Lyons KE (2005) Preoperative clinical predictors of response to bilateral subthalamic stimulation in patients with Parkinson’s disease. Stereotact Funct Neurosurg 832–3:80–83
Isaias I U, Alterman R L, and Tagliati M (2008) Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain 131Pt 7: 1895–902.
Vasques X, Cif L, Gonzalez V, Nicholson C, Coubes P (2009) Factors predicting improvement in primary generalized dystonia treated by pallidal deep brain stimulation. Mov Disord 246:846–853
Vasques X, Cif L, Hess O, Gavarini S, Mennessier G, Coubes P (2009) Prognostic value of globus pallidus internus volume in primary dystonia treated by deep brain stimulation. J Neurosurg 1102:220–228
Hemm S, Wårdell K (2010) Stereotactic implantation of deep brain stimulation electrodes: a review of technical systems, methods and emerging tools. Med Biol Eng Comput 487:611–624
Åström M, Zrinzo LU, Tisch S, Tripoliti E, Hariz MI, Wårdell K (2009) Method for patient-specific finite element modeling and simulation of deep brain stimulation. Med Biol Eng Comput 471:21–28
Butson CR, Cooper SE, Henderson JM, McIntyre CC (2007) Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 342:661–670
Åström M, Tripoliti E, Hariz MI, Zrinzo LU, Martinez-Torres I, Limousin P, Wårdell K (2010) Patient-specific model-based investigation of speech intelligibility and movement during deep brain stimulation. Stereotact Funct Neurosurg 884:224–233
Nicholson PW (1965) Specific impedance of cerebral white matter. Exp Neurol 134:386–401
Sotiropoulos SN, Steinmetz PN (2007) Assessing the direct effects of deep brain stimulation using embedded axon models. J Neural Eng 42:107–119
Tuch DS, Wedeen VJ, Dale AM, George JS, Belliveau JW (2001) Conductivity tensor mapping of the human brain using diffusion tensor MRI. Proc Natl Acad Sci USA 9820:11697–11701
Tuch DS, Wedeen VJ, Dale AM, George JS, Belliveau JW (1999) Conductivity mapping of biological tissue using diffusion MRI. Ann N Y Acad Sci 888:314–316
Haueisen J, Tuch DS, Ramon C, Schimpf PH, Wedeen VJ, George JS, Belliveau JW (2002) The influence of brain tissue anisotropy on human EEG and MEG. Neuroimage 151:159–166
Walckiers G, Fuchs B, Thiran JP, Mosig JR, Pollo C (2010) Influence of the implanted pulse generator as reference electrode in finite element model of monopolar deep brain stimulation. J Neurosci Methods 1861:90–96
Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 2301:77–87
Chaturvedi A, Butson CR, Lempka SF, Cooper SE, McIntyre CC (2010) Patient-specific models of deep brain stimulation: influence of field model complexity on neural activation predictions. Brain Stimul 32:65–67
Andreuccetti D, Fossi R, Petrucci C (2005) Dielectric properties of body tissue. Italian National Research Council, Institute for Applied Physics, Florence. http://niremf.ifac.cnr.it/tissprop/
Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 812:106–116
Cheng D K (1989) Field and Wave Electromagnetics. Vol. ISBN 0-201-52820-7. 1989: Addison-Wesley Publishing Company Inc., New York
Kindlmann G (2004) Superquadric tensor glyphs. In: Proceedings IEEE TVCG/EG symposium on visualization, pp 147–154
Ennis DB, Kindlman G, Rodriguez I, Helm PA, McVeigh ER (2005) Visualization of tensor fields using superquadric glyphs. Magn Reson Med 531:169–176
Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R (2002) Processing and visualization for diffusion tensor MRI. Med Image Anal 62:93–108
Rattay F (1986) Analysis of models for external stimulation of axons. IEEE Trans Biomed Eng 3310:974–977
Butson CR, McIntyre CC (2005) Tissue and electrode capacitance reduce neural activation volumes during deep brain stimulation. Clin Neurophysiol 11610:2490–2500
Butson CR, Maks CB, McIntyre CC (2006) Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol 1172:447–454
Yousif N, Bayford R, Wang S, Liu X (2008) Quantifying the effects of the electrode-brain interface on the crossing electric currents in deep brain recording and stimulation. Neuroscience 1523:683–691
Kuncel AM, Grill WM (2004) Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol 11511:2431–2441
Kuncel AM, Cooper SE, Grill WM (2008) A method to estimate the spatial extent of activation in thalamic deep brain stimulation. Clin Neurophysiol 1199:2148–2158
Hemm S, Mennessier G, Vayssiere N, Cif L, El Fertit H, Coubes P (2005) Deep brain stimulation in movement disorders: stereotactic coregistration of two-dimensional electrical field modeling and magnetic resonance imaging. J Neurosurg 1036:949–955
Mikos A, Bowers D, Noecker AM, McIntyre CC, Won M, Chaturvedi A, Foote KD, Okun (2011) Patient-specific analysis of the relationship between the volume of tissue activated during DBS and verbal fluency. Neuroimage 54(Suppl 1):S238–S246
Frankemolle AM, Wu J, Noecker AM, Voelcker-Rehage C, Ho JC, Vitek JL, McIntyre CC, Alberts JL (2010) Reversing cognitive-motor impairments in Parkinson’s disease patients using a computational modelling approach to deep brain stimulation programming. Brain 133(Pt 3):746–761
Geddes LA, Baker LE (1967) The specific resistance of biological material–a compendium of data for the biomedical engineer and physiologist. Med Biol Eng 53:271–293
Schwan HP, Kay CF (1957) The conductivity of living tissues. Ann N Y Acad Sci 656:1007–1013
Latikka J, Kuurne T, Eskola H (2001) Conductivity of living intracranial tissues. Phys Med Biol 466:1611–1616
Hemm S, Vayssiere N, Mennessier G, Cif L, Zanca M, Ravel P, Frerebeau P, Coubes P (2004) Evolution of brain impedance in dystonic patients treated by GPi electrical stimulation. Neuromodulation 7(2):75
Gabriel C, Gabriel S, Corthout E (1996) The dielectric properties of biological tissues: I literature survey. Phys Med Biol 4111:2231–2249
Gabriel S, Lau RW, Gabriel C (1996) The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol 4111:2251–2269
Gabriel S, Lau RW, Gabriel C (1996) The dielectric properties of biological tissues: III parametric models for the dielectric spectrum of tissues. Phys Med Biol 4111:2271–2293
McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL (2004) Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol 1153:589–595
Landman BA, Wan H, Bogovic JA, Bazin PL, Prince JL (2010) Resolution of crossing fibers with constrained compressed sensing using traditional diffusion tensor MRI. Proc Soc Photo Opt Instrum Eng 7623:76231H
Wei XF, Grill WM (2005) Current density distributions, field distributions and impedance analysis of segmented deep brain stimulation electrodes. J Neural Eng 24:139–147
Johnson MD, McIntyre CC (2008) Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol 1005:2549–2563
Grant PF, Lowery MM (2009) Electric field distribution in a finite-volume head model of deep brain stimulation. Med Eng Phys 319:1095–1103
Lemaire JJ, Coste J, Ouchchane L, Caire F, Nuti C, Derost P, Cristini V, Gabrillargues J, Hemm S, Durif F, Chazal J (2007) Brain mapping in stereotactic surgery: a brief overview from the probabilistic targeting to the patient-based anatomic mapping. Neuroimage 37(Suppl 1):S109–S115
Åström M, Johansson JD, Hariz MI, Eriksson O, Wårdell K (2006) The effect of cystic cavities on deep brain stimulation in the basal ganglia: a simulation-based study. J Neural Eng 32:132–138
Acknowledgments
The authors would like to thank Simone Hemm-Ode, PhD, Göran Salerud, PhD, and Mats Andersson, PhD, for valuable input, and Associate professor Eva Enqvist, for statistical contributions. This study was financially supported by the Swedish Foundation for Strategic Research (SSF), Swedish Research Council (VR, grant number 621-2008-3013), and Swedish Governmental Agency for Innovation Systems (VINNOVA, group grant number 311-2006-7661).
Conflict of interest
None of the authors reported any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Åström, M., Lemaire, JJ. & Wårdell, K. Influence of heterogeneous and anisotropic tissue conductivity on electric field distribution in deep brain stimulation. Med Biol Eng Comput 50, 23–32 (2012). https://doi.org/10.1007/s11517-011-0842-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-011-0842-z