Abstract
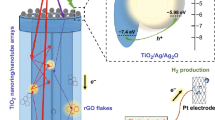
TiO2 nanotube arrays (TiO2 NTs) were fabricated by anodic oxidation and then Ag nanoparticles (Ag NPs) were assembled in TiO2 NTs (Ag/TiO2 NTs) by microwave-assisted chemical reduction. The samples were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), photoluminescence spectrum (PL), UV–vis absorption spectrum (UV–vis), and Raman spectrum, respectively. The results showed that Ag NPs were well dispersed on the surface of TiO2 NTs with metallic state. The surface plasmon resonance (SPR) effect of Ag NPs could extend the visible light response and enhance the absorption capacity of TiO2. Furthermore, Ag NPs could also restrain the recombination of photo-generated electron–hole pairs of TiO2 NTs efficiently. The methylene blue photodegradation experiment proved that the SPR phenomenon had an effect on photoreaction enhancement. The results of photocatalytic water splitting indicated that Ag/TiO2 NTs samples had better photocatalytic performance than pure TiO2 NTs. The corresponding hydrogen evolution rate of Ag/TiO2 NTs prepared with 0.002 M AgNO3 solution was 3.3 times as that of pure TiO2 NTs in the test condition. Additionally, the mechanism of catalyst activity enhanced by SPR effect was proposed.










Similar content being viewed by others
References
Lee J, Jho JY (2011) Sol Energy Mater Sol Cells 95:3152–3156. doi:10.1016/j.solmat.2011.06.046
Park H, Kim WR, Jeong HT, Lee JJ, Kim HG, Choi WY (2011) Sol Energy Mater Sol Cells 95:184–189. doi:10.1016/j.solmat.2010.02.017
Park JH, Kim JY, Kim JH, Choi CJ, Kim H, Sung YE, Ahn KS (2011) J Power Sources 196:8904–8908. doi:10.1016/j.jpowsour.2011.06.063
Lu N, Zhao H, Li J, Quan X, Chen S (2008) Sep Purif Technol 62:668–673. doi:10.1016/j.seppur.2008.03.021
Quan X, Ruan X, Zhao H, Chen S, Zhao Y (2007) Environ Pollut 147:409–414. doi:10.1016/j.envpol.2006.05.023
Xu J, Ao Y, Chen M, Fu D (2010) Appl Surf Sci 256:4397–4401. doi:10.1016/j.apsusc.2010.02.037
Wender H, Feil AF, Diaz LB, Riberio CS, Machado GJ, Migowski P, Weibel DE, Dupont J, Teixera SR (2011) ACS Appl Mater Interfaces 3:1359–1365. doi:10.1021/la9020146
Meng Q, Wang J, Xie Q, Dong H, Li X (2011) Catal Today 165:145–149. doi:10.1016/j.cattod.2010.11.086
Sun Y, Wang G, Yan K (2011) Int J Hydrogen Energy 36:15502–15508. doi:10.1016/j.ijhydene.2011.08.112
Lin S, Li D, Wu J, Li X, Akbar SA (2011) Sens Actuators 156:505–509. doi:10.1016/j.snb.2011.02.046
Galstyan V, Comini E, Faglia G, Vomiero A, Brisotto M, Bontempi E, Sberveglieri G (2011) Procedia Eng 25:757–760. doi:10.1016/j.proeng.2011.12.186
Li J, Lu N, Quan X, Chen S, Zhao H (2008) Ind Eng Chem Res 47:3804–3808. doi:10.1021/ie0712028
Lai Y, Huang J, Zhang H, Subramaniam VP, Tang Y, Gong DG, Sundar L, Sun L, Chen Z, Lin C (2010) J Hazard Mater 184:855–863. doi:10.1016/j.jhazmat.2010.08.121
Su Y, Chen S, Quan X, Zhang Y (2008) Appl Surf Sci 255:2167–2172. doi:10.1016/j.apsusc.2008.07.053
Zhang J, Tang C, Bang J (2010) Electrochem Commun 12:1124–1128. doi:10.1016/j.elecom.2010.05.046
Shin K, Seok S, Im SH, Park JH (2010) Chem Commun 46:2385–2387. doi:10.1039/B923022J
Kang Q, Liu S, Yang L, Cai Q, Grimes CA (2011) ACS Appl Mater Interfaces 3:746–749. doi:10.1021/am101086t
Paramasivam I, Macak JM, Schmuki P (2008) Electrochem Commun 10:71–75. doi:10.1016/j.elecom.2007.11.001
Liang Y, Wang C, Kei CC, Hsueh YC, Cho WH, Perng TP (2011) J Phys Chem C 115:9498–9502. doi:10.1021/jp202111p
Song Y, Gao Z, Schmuki P (2011) Electrochem Commun 13:290–293. doi:10.1016/j.elecom.2011.01.006
Logar M, Jančar B, Šturm S, Suvorov D (2010) Langmuir 26:12215–12224. doi:10.1021/la101124q
Awazu K, Fujimaki M, Rockstuhl C, Tominaga J, Murakami H, Ohki Y, Yoshida N, Watanabe T (2008) J Am Chem Soc 130:1676–1680. doi:10.1021/ja076503n
Christopher P, Ingram DB, Linic S (2010) J Phys Chem C 114:9173–9177. doi:10.1021/jp101633u
Chen JJ, Wu JCS J, Wu PC, Tsai DP (2011) J Phys Chem C 115:210–216. doi:10.1021/jp1074048
Yu J, Dai G, Huang B (2009) J Phys Chem C 113:16394–16401. doi:10.1021/jp905247j
Naya S, Inoue A, Tada H (2010) J Am Chem Soc 132:6292–6293. doi:10.1021/ja101711j
Periyat P, Leyland N, McCormack DE, Colreavy J, Corr D, Pillai SC (2010) J Mater Chem 20:3650–3655. doi:10.1039/B924341K
Liang Y, Cui Z, Zhu S, Liu Y, Yang X (2011) J Catal 278:276–287. doi:10.1016/j.jcat.2010.12.011
Zhou J, Zhang Y, Zhao X, Ray AK (2006) Ind Eng Chem Res 45:3503. doi:10.1021/ie051098z
Sun L, Li J, Wang C, Li S, Lai Y, Chen H, Lin C (2008) J Hazard Mater 171:1045–1050. doi:10.1016/j.jhazmat.2009.06.115
Amendola V, Bakr OM, Stellacci F (2010) Plasmonics 5:85–97. doi:10.1007/s11468-009-9120-4
Wang C, Liu C, Liu Y, Zhang Z (1999) Appl Surf Sci 147:52–57. doi:10.1016/S0169-4332(99)00117-8
Zhou Y, Wang C, Liu H (1999) Mater Sci Eng B 67:95–98. doi:10.1016/S0921-5107(99)00316-5
Yang Y, Chang C, Idriss H (2006) Appl Catal B: Environ 67:217–222. doi:10.1016/j.apcatb.2006.05.007
Acknowledgments
The authors greatly acknowledge the National Natural Science Foundation of China (No.21176199), the Research Fund for the Doctoral Program of Higher Education (No.20096101110013), and the Natural Science Foundation of Shannxi Province (No.2010JZ002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, F., Hu, X., Fan, J. et al. Photocatalytic Activity of Ag/TiO2 Nanotube Arrays Enhanced by Surface Plasmon Resonance and Application in Hydrogen Evolution by Water Splitting. Plasmonics 8, 501–508 (2013). https://doi.org/10.1007/s11468-012-9418-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-012-9418-5