Abstract
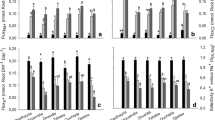
To evaluate genotypic difference in antioxidative ability and salt tolerance in poplars, the authors investigated the effects of increasing content of soil NaCl on salt concentration in leaves, superoxide dismutase (SOD) and peroxidase (POD) activities, malondialdehyde (MDA) content, and membrane permeability (MP) in Populus euphratica Oliv., P. popularis “35–44,” and P. × euramericana cv. I-214 (hereafter abbreviated as P. cv. I-214). Na+ and Cl− concentrations in leaves of P. popularis increased markedly over the increase of the duration of exposure to salinity, and culminated after 28 days of salt stress. SOD and POD activities declined correspondingly, followed by significant increases of MDA and MP, and leaf injury was finally observed. Compared with P. popularis, leaf Na+ and Cl− in P. cv. I-214 exhibited a trend similar to P. popularis, but a lower salt-induced increase of MDA and permeability was observed and lighter leaf necrosis occurred. In contrast to P. popularis and P. cv. I-214, SOD and POD activities in P. euphratica leaves increased rapidly at the beginning of salt stress with a light soil NaCl concentration of 58.5 mmol/L. Furthermore, salt ion concentration, MDA content, and MP in P. euphratica leaves did not increase significantly during 28 days of increasing salt stress. Therefore, the increase in MP in P. popularis and P. cv. I-214 had a close relationship with a salt buildup in leaves under increasing salt stress. Salt-induced declines of SOD and POD activities might accelerate lipid peroxide and consequently resulted in ion leakage. P. euphratica rapidly activated antioxidant enzymes after the onset of salt stress, which might reduce the accumulation of reactive oxygen species and the subsequent acceleration of lipid peroxide. P. euphratica leaves exhibited a higher capacity to exclude salt in a longer period of increasing salinity, thus limited salt-induced lipid peroxide and MP, which contributed to membrane integrity maintenance and salt tolerance of P. euphratica.
Similar content being viewed by others
References
Chen S.-Y., Injury of membrane lipid peroxidation to plant cell, Plant Physiol. Commun., 1991, 27(2): 84–90
Imlay J.-A. and Linn S., DNA damage and oxygen radical toxicity, Science, 1998, 240: 1,302–1,309
Byrd S., Reins D. and Doetsch P.-W., Effects of oxidative DNA damage on transcription by RNA polymerases, Free Radic. Biol. Med., 1990, 9(1): 47–57
Foyer C.-H., Descourvieres P. and Kunert K.-J., Protection against oxygen radicals: an important defense mechanism studied in transgenic plants, Plant Cell Environ., 1994, 17: 507–523
Tsugane K., Koboyashi K. and Niwa Y., A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification, Plant Cell, 1999, 11: 1,195–1,206
Ma H.-C. and Wang S.-S., The stability of the membrane and metabolic response of P. euphratica under salt stress, J. Southwest For. Coll., 1998, 18(1): 15–22
Chen S., Li J., Wang S. and Hüttermann A., Salt, nutrient uptake and transport and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl, Trees, 2001, 15(3): 186–194
Chen S., Li J. and Fritz E., Sodium and chloride distribution in roots and transport in three poplar genotypes under increasing NaCl stress, For. Ecol. Manag., 2002, 168(1–3): 217–230
Giannopolits C.-N. and Ries S.-K., Superoxide dismutases: occurrence in higher plants, Plant Physiol., 1977, 59: 309–314
Kochba J., Lavce S. and Spiegel R.-P., Difference in peroxidase activity and isoenzymes in embryogenic orange ovular callus lines, Plant Cell Physiol., 1977, 18: 463–467
Heath R.-L. and Pacher L., Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation, Arch. Biochem. Biophys., 1968, 125: 189–198
Fadzilla N.-M., Finch R.-P. and Burdon R.-H., Salinity, oxidative stress and antioxidant responses in shoot culture of rice, J. Exp. Bot., 1997, 48: 325–331
Hernández J.-A., Corpass F.-J. and Gome M., Salt-induced oxidative stress mediated by active oxygen species in pea leaf mitochondria, Physiol. Plant., 1993, 89: 103–110
Smirnoff N., Antioxidant Systems and Plant Response to the Environment. In: Smirnoff N. (ed.) Environment and Plant Metabolism: Flexibility and Acclimation, Oxford: Bios Scientific Press, 1995: 217–243
Bandeoglue E., Eyidogan F. and Yücel M, Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress, Plant Growth Regul., 2004, 42: 69–77
Abed S. and Peter M.-N., Exogenous ascorbic (vitamin C) increases resistance to salt stress and reduces lipid peroxidation, J. Exp. Bot., 2001, 52(364): 2,207–2,211
Halliwell B. and Gutteridge J.-M.-C., Free Radicals in Biology and Medicine, 3rd edn., Oxford: Oxford University Press, 1999, 936–975
Liu Y.-L., Mao C.-L. and Wang L.-J., Advances in salt tolerance in plants, Commun. Plant Physiol., 1987, 23: 1–7
Ruth G.-A., Neval E. and Lenwood S.-H., Role of superoxide dismutases in controlling oxidative stress in plants, J. Exp. Bot., 2002, 53(372): 1n331–1n341
Asada K. and Takahashi M., Production and scavenging of active oxygen in photosynthesis. In: Kyle D.-J., Osmond C.-B., Arntzen C.-J. (eds.) Photoinhibition, Amsterdam: Elsevier Science Publishers, 1987: 227–287
Hernández J.-A., Olmo E. and Corpass F.-J., Salt-induced oxidative stress in chloroplast of pea plants, Plant Sci., 1995, 105: 151–167
Shigeru S., Takahiro I. and Masahiro T., Regulation and function of ascorbate peroxidase isoenzymes, J. Exp. Bot., 2002, 53(372): 1,305–1,319
Li M., Wang G.-X., Lin J.-S., Application of external calcium in improving the PEG-induced water stress tolerance in liquorice cells, Bot. Bull. Acad. Sin., 2003, 44: 275–284
Liang Y.-C., Chen Q. and Liu Q., Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.), J. Plant Physiol., 2003, 160: 1,157–1,164
Lutts S., Kinet J.-M. and Bouharmont J., NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance, Ann. Bot., 1996, 78: 389–398
Chen S., Li J. and Fritz E., Osmotic stress and ion-specific effects on xylem abscisic acid the relevance to salinity tolerance in poplar, J. Plant Growth Regul., 2002, 21(3): 224–233
Anderson M.-D., Prasad T.-K. and Martin B.-A., Differential gene expression in chilling-acclimated maize seedlings and evidence for the involvement of abscisic acid in chilling tolerance, Plant Physiol., 1994, 105: 331–339
Bueno P., Piqueras A. and Kurepa J., Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures, Plant Sci., 1998, 138: 27–34
Guan L. and Scandalios J.-G., Effects of the plant growth regulator abscisic acid and high osmoticum on the developmental expression of the maize catalase genes, Physiol. Plant., 1998, 104: 413–422
Guan L. and Scandalios J.-G., Two structurally similar maize cytosolic superoxide dismutase genes, Sod4 and Sod4A, respond differentially to abscisic acid and high osmoticum, Plant Physiol., 1998, 117: 217–224
Guan L.-M., Zhao J. and Scandalios J.-G., Cis-element and transfactors that regulate expression of maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response, Plant J., 2000, 22: 87–95
Prasad T.-K., Anderson M.-D. and Stewart C.-R., Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings, Plant Physiol., 1994, 105: 619–627
Gong M., Li Y. and Chen S.-Z., Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems, J. Plant Physiol., 1998, 153: 488–496
Bellaire B.-A., Carmody J. and Gossett D.-R., Involvement of abscisic acid-dependent and -independent pathways in the upregulation of antioxidant enzyme activity during NaCl stress in cotton callus tissue, Free Radic. Res., 2000, 33: 531–545
Larkindale J. and Knight M.-R., Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid, Plant Physiol., 2002, 128: 682–695
Jiang M. and Zhang J., Role of abscisic acid in water stress induced antioxidant defense in leaves of maize seedlings, Free Radic. Res., 2002, 36: 1,001–1,015
Jiang M. and Zhang J., Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves, J. Exp. Bot., 2002, 53: 2,401–2,410
Jiang M. and Zhang J., Involvement of plasma membrane NADPH oxidase in abscisic acid and water stress-induced antioxidant defense in leaves of maize seedlings, Planta, 2002, 215: 1,022–1,030
Xu X., Mao G.-L. and Li S.-H., Effect of salt stress and abscisic acid on membrane-lipid peroxidation and resistant-oxidation enzyme activities of Lycium barbarum callus, Acta Bot. Boreal-occident. Sin., 2003, 23(5): 745–774
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Journal of Beijing Forestry University, 2005, 27(3) (in Chinese)
About this article
Cite this article
Wang, R., Chen, S., Ma, H. et al. Genotypic Differences in Antioxidative Stress and Salt Tolerance of Three Poplars Under Salt Stress. Front. Forest. China 1, 82–88 (2006). https://doi.org/10.1007/s11461-005-0019-8
Issue Date:
DOI: https://doi.org/10.1007/s11461-005-0019-8