Abstract
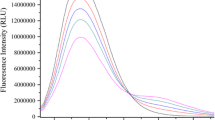
This work attempts to calculate the binding-site number using fluorescence spectroscopic method with bovine serum albumin (BSA) and Indo-1 as protein and ligand models, respectively. The method for calculating the binding-site number in BSA for Indo-1 was developed based on the relationships between changes in Indo-1 fluorescence intensity and the analytical concentration of BSA. The interaction between BSA with Indo-1 was investigated comprehensively using fluorescence techniques as well as fluorescence resonance energy transfer, and the thermodynamic parameters were calculated according to the effect of enthalpy on temperature. Three binding sites in BSA for Indo-1 were revealed, and the distances from Trp212 in BSA to the three binding sites were 2.93, 2.57 and 2.40 nm, respectively. It was also proven that Indo-1 embedded into the three hydrophobic cavities of BSA by hydrophobic association. This paper provides a reference on calculating the binding-site number in proteins for ligands and studying their interactions by fluorescence spectroscopic methods. In fluorescent quenching experiments, fluorescence changes were automatically recorded in real time by combining the Microlab 500 Series Dispenser and PTI fluorescence apparatus.
Similar content being viewed by others
References
Lakowicz J R. Principles of Fluorescence Spectroscopy (2nd Edition), Kuwer Academic/Plenum Publisher, New York, 1999, a: p1, b: p239, c: p248, d: p240
Romer J, Bichkel M H. Method to estimate binding constants at variable protein concentrations. J Pharm Pharmacol, 1979, 31: 7–11
Bhattacharyya M, Chaudhuri U, Poddar R K. Evidence for Cooperative Binding of Chloropromazine With Hemoglobin: Equilibrium Dialysis, Fluorescence Quenching and Oxygen Release Study. Biochem Biophys Res Commun, 1990, 167: 1146–1153
Zhao H, Su W, Luo Y H, Ji Y H, Li ZC, Jiu H F, Liang H, Chen B, Zhang Q J. Rectification of excitation with bathochromic shift induced by intense absorption of organic ligands during emission. Spectrochim. Acta A, 2006, 65: 846–851
Subbiah D, Ashok K M. Fluorescence spectroscopic study of serum albumin-bromadiolone interaction: fluorimetric determination of bromadiolone. J Pharm Biomed Anal, 2005, 38: 556–563
Hirshfield K M, Toptygin D, Grandhige G, Kim H, Packard B Z, Brand L. Steady-state and time-resolved fluorescence measurements for studying molecular interactions: interaction of a calcium-binding probe with proteins. Biophys Chem, 1996, 62: 25–38
Richieri G V, Anel A, Kleinfeld A M. Interaction of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry, 1993, 32: 7574–7580
Olson M K, Hollingworth S, Baylor S M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J, 1988, 54: 1089–1104
Kurebayashi N, Harkins A B, Baylor S M. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J, 1993, 64: 1934–1960
Cantor C R, Schimmel P R. Biophysical Chemistry, Part III: The Behavior of Biological Macromolecules (Freeman, San Francisco), 1980, pp. 849–886
Yang Man-man, Yang Pin, Zhang Li-wei. Studies on the interaction of caffeinic drugs with albumin by fluorescence methods. Chinese Sci Bull, 1994, 39(1): 31–35
Vallner J J. Binding of drugs by albumin and plasma-protein. J Pharm Sci, 1997, 66: 447–465
Carter D C, Ho J X. Structure of serum albumin. Adv Protein Chem, 1994, 45: 153–203
Ikenouchi H, Peeters G A, Barry W H. Evidence that binding of indo-1 to cardiac myocyte protein does not markedly change Kd for Ca2+ Cell Calcium. Cell Calcium, 1991, 12: 415–422
Owen C S, Shuler R L. Spectral evidence for non-calcium interactions of intracellular indo-1. Biochem Biophys Res Commun, 1989, 163: 328–333
Bancel F, Salmon J M, Vigo J, Viallet P. Microspectrofluorometry as a tool for investigation of noncalcium interactions of indo-1. Cell Calcium, 1992, 13: 59–68
Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem, 1985, 260(6): 3440–3450
Ribou A C, Vigo J, Viallet P. Interaction of a protein, BSA, and a fluorescent probe, Mag-Indo-1, influence of EDTA and calcium on the equilibrium. J M Salmon, Biophys Chem, 1999, 81: 179–189
Morelle B, Salmon J M, Vigo J, Viallet P. Measurement of intracellular magnesium concentrations in 3T3 fibroblasts with the. fluorescent indicator MagIndo-1. Anal Biochem, 1994, 218: 170–176
Tian J, Liu J, Tian X, Hu Z, Chen X. Study of the interaction of kaernpferol with bovine serum albumin. J Mol Struct, 2004, 691: 197–202
Philip D, Ross Sabramarian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20(1981) 3096–3102
Krang-hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol Rev, 1981, 33(1): 17–53
Wei Y J, Li K A, Tong S Y. The interaction of Bromophenol blue with proteins in acidic solution. Talanta, 1996, 43: 1–10
Yang B, Spectral studies on the combination of lanthanon ion with partner albumin. Chem J Chinese U, 1998, 19(7): 1057–1061
Chi Y, Zhuang J, Li N, Li K, Tong S. Studies on the interaction mechanism of bovine serum albumin with zinc reagent. Chem J Chinese U, 1999, 20(11): 1697–1702
Förster T, Sinaanoglu, (Eds.). Modern Quantum Chemistry, vol. 3. Academic Press, New York, 1996, p. 93
Majoul I, Straub M, Duden R, Hell S W, Söling H D. Fluorescence resonance energy transfer analysis of protein-protein interactions in single living cells by multifocal multiphoton microscopy. Mol. Biotech, 2002, 82: 267–277
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Chemical Journal of Chinese Universities, 2007, 28(2): 227–233 [译自: 高等学校化学学报]
About this article
Cite this article
Bai, H., Yang, C. & Yang, X. Interaction between bovine serum albumin and Indo-1 using fluorescence spectroscopic method. Front. Chem. China 3, 105–111 (2008). https://doi.org/10.1007/s11458-008-0013-4
Issue Date:
DOI: https://doi.org/10.1007/s11458-008-0013-4