Abstract
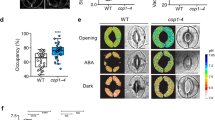
Hydrogen sulfide (H2S) is a newly discovered gaseous signaling molecule and involved in ethylene and ABA-induced stomatal closure. As an important factor, extracellular ATP (eATP) was believed to participate in regulation of stomatal closing. However, the mechanism by which eATP mediates H2S-regulated stomatal closure remains unclear. Here, we employed Arabidopsis wild-type and mutant lines of ATP-binding cassette transporters (Atmrp4, Atmrp5 and their double mutant Atmrp4/5) to study the function of eATP in H2S-regulated stomatal movement. Our results indicated that H2S affected stomatal closing through stimulating guard cell outward K+ current. Moreover, we found that H2S induced eATP generation by regulating the activity of an ABC transporter. The inhibitor of ABC transporters, glibenclamide (Gli), could impair H2S-regulated stomatal closure and reduce H2S-dependent eATP accumulation in Atmrp4 and Atmrp5 mutants. In addition, the promotion effect of H2S on outward K+ currents was diminished in Atmrp4/5 double mutant. Our data suggested that hydrogen peroxide (H2O2) is required for H2S-induced stomatal closure, and the production of H2O2 is regulated by eATP via NADPH oxidase. Based on this work, we conclude that H2S-induced stomatal closure requires ABC transporter-dependent eATP production and subsequent NADPH oxidase-dependent H2O2 accumulation.






Similar content being viewed by others
References
Kim TH, Böhmer M, Hu H et al (2001) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61:561–591
Fan LM, Zhao Z, Assmann SM (2004) Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7:537–546
García-Mata C, Lamattina L (2013) Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201:66–73
Kajimura M, Fukuda R, Bateman RM et al (2010) Interaction of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO and H2S gas biology. Antioxid Redox Signal 13:157–192
Tan BH, Wong PT, Bian JS (2010) Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem Int 56:3–10
Lisjak M, Teklić T, Wilson ID et al (2013) Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ 36:1607–1616
García-Mata C, Lamattina L (2010) Hydrogen sulphide a novel gasotransmitter involved in guard cell signaling. New Phytol 188:977–984
Hou ZH, Wang LX, Liu J et al (2013) Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. J Integr Plant Biol 55:277–289
Liu J, Hou LX, Liu GH et al (2011) Hydrogen sulfide induced by nitric oxide mediates ethylene-induced stomatal closure of Arabidopsis thaliana. Chin Sci Bull 56:3547–3553
Lisjak M, Srivastava N, Teklić T et al (2010) A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48:931–935
Lisjak M, Teklić T, Wilson ID et al (2011) Hydrogen sulfide effects on stomatal apertures. Plant Signal Behav 6:1444–1446
Ye Q, Hou ZH, Liu J et al (2011) H2O2 involvement in H2S-induced stomatal closure of Arabidopsis thaliana L. Plant Physiol J 47:1195–1200 (in Chinese)
Kim SY, Sivaguru M, Stacey G (2006) Extracellular ATP in plants, visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol 142:984–992
Chivasa S, Ndimba BK, Simon WJ et al (2005) Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 17:3019–3034
Clark G, Wu M, Wat N et al (2010) Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Mol Biol 74:423–435
Jeter CR, Tang W, Henaff E et al (2004) Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16:2652–2664
Weerasinghe RR, Swanson SJ, Okada SF et al (2009) Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett 583:2521–2526
Thomas C, Rajagopal A, Windsor B et al (2000) A role for ectophosphatase in xenobiotic resistance. Plant Cell 12:519–533
Klein M, Perfus-Barbeoch L, Frelet A et al (2003) The plant multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signalling and water use. Plant J 33:119–129
Gaedeke N, Klein M, Kolukisaoglu U et al (2001) The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J 20:1875–1887
Bodin P, Burnstock G (2001) Purinergic signaling ATP release. Neurochem Res 26:959–969
Clark G, Fraley D, Steinebrunner I et al (2011) Extracellular nucleotides and apyrases regulate stomatal aperture in Arabidopsis. Plant Physiol 156:1740–1753
Clark G, Darwin C, Mehta V et al (2013) Effects of chemical inhibitors and apyrase enzyme further document a role for apyrases and extracellular ATP in the opening and closing of stomates in Arabidopsis. Plant Signal Behav 8:e26093
Assmann SM (1993) Signal transduction in guard cells. Annu Rev Cell Biol 9:345–375
Zhao X, Qiao XR, Yuan J et al (2012) Nitric oxide inhibits blue light-induced stomatal opening by regulating the K+ influx in guard cells. Plant Sci 184:29–35
Lemtiri-Chlieh F, Berkowitz GA (2004) Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J Biol Chem 279:35306–35312
Zhang W, Nilson SE, Assmann SM (2008) Isolation and whole-cell patch clamping of Arabidopsis guard cell protoplasts. CSH Protoc. doi:10.1101/pdb.prot5014
Zhang W, He SY, Assmann SM (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56:984–996
Hou ZH, Che YM, Wang LX et al (2012) H2S functions downstream of H2O2 in mediating ethylene-induced stomatal closure in Arabidopsis thaliana. Plant Physiol J 48:1193–1199 (in Chinese)
Klein M, Geisler M, Suh SJ et al (2004) Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. Plant J 39:219–236
Suh SJ, Wang YF, Frelet A et al (2007) The ATP binding cassette transporter AtMRP5 modulates anion and calcium channel activities in Arabidopsis guard cells. J Biol Chem 282:1916–1924
Wu SJ, Liu YS, Wu JY (2008) The signaling role of extracellular ATP and its dependence on Ca2+ flux in elicitation of Salvia miltiorrhiza hairy root cultures. Plant Cell Physiol 49:617–624
Hao LH, Wang WX, Chen C et al (2012) Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein α subunit and reactive oxygen species. Mol Plant 5:852–864
Lü D, Wang W, Miao C et al (2013) ATHK1 acts downstream of hydrogen peroxide to mediate ABA signaling through regulation of calcium channel activity in Arabidopsis guard cells. Chin Sci Bull 58:336–343
Hosy E, Vavasseur A, Mouline K et al (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA 100:5549–5554
Pandey S, Zhang W, Assmann SM (2007) Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett 581:2325–2336
Sato A, Sato Y, Fukao Y et al (2009) Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem J 424:439–448
Becker D, Hoth S, Ache P et al (2003) Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett 554:119–126
Buckler KJ (2012) Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflügers Arch-Eur J Physiol 463:743–754
Takahashi Y, EbisuY Kinoshita T et al (2013) bHLH transcription factors that facilitate K+ uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Sci Signal 6:ra48
Munemasa S, Oda K, Watanabe-Sugimoto M et al (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells, specific impairment of ion channel activation and second messenger production. Plant Physiol 143:1398–1407
Vahisalu T, Kollist H, Wang YF et al (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signaling. Nature 452:487–491
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31170237), the National Key Laboratory Program of China Agricultural University (SKLPPBKF11001) and Shandong Taishan Scholar Program. We thank Prof. Dong Chunhai and Dr. Qian Ma (Qingdao Agricultural University) for helpful suggestions and critical reading and polishing of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Wang, L., Ma, X., Che, Y. et al. Extracellular ATP mediates H2S-regulated stomatal movements and guard cell K+ current in a H2O2-dependent manner in Arabidopsis . Sci. Bull. 60, 419–427 (2015). https://doi.org/10.1007/s11434-014-0659-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-014-0659-x