Abstract
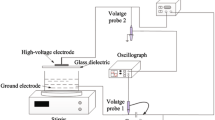
The degradation of an azo dye, acid orange 7 (AO7), caused by different high voltage pulsed electrical discharge modes (spark, streamer and corona discharge) induced by the various initial conductivities was investigated. A new type of pulsed high voltage source with thyratron switch and Blumlein pulse forming net (BPFN) was used. The typical discharge waveforms of voltage, current, power, pulse energy and the pictures of spark, streamer and corona discharge modes were presented. The results indicated that pulsed electrical discharges led to complete decolorization and substantial decrease of the chemical oxygen demand (COD) of the dye solution. The main intermediate products were monitored by GC-MS. The discharge modes changed from spark to streamer and to corona discharge, and the streamer length decreased with the liquid conductivity increasing. At a constant input power, the peak voltage, peak current, peak power and energy per pulse of the three discharge modes ranked in the following order: spark > streamer > corona. The effective energy transfer efficiency of AO7 removal was higher for spark discharge (57.2%) than for streamer discharge (40.4%) and corona discharge (27.6%). Moreover, the energy utilization efficiency of AO7 removal for spark discharge was 1.035×109 mol/J, and for streamer and corona discharge they were 0.646×10−9 and 0.589×10−9 mol/J. Both the energy transfer efficiency and the energy utilization efficiency of spark discharge were the highest.
Similar content being viewed by others
References
Zhang X W, Zhou M H, Lei L C. TiO2 photocatalyst deposition by MOCVD on activated carbon. Carbon, 2006, 44: 325–333
Zhou M H, Dai Q Z, Lei L C, et al. Long life modified lead dioxide anode for organic wastewater treatment: Electrochemical characteristics and degradation mechanism. Environ Sci Technol, 2005, 39: 363–370
Du Y X, Zhou M H, Lei L C. Role of the intermediates in the degradation of phenolic compounds by Fenton-like process. J Hazard Mater, 2006, 136: 859–865
Kishimoto N, Morita Y, Tsuno H, et al. Advanced oxidation effect of ozonation combined with electrolysis. Water Res, 2005, 39: 4661–4672
Qamar M, Muneer M, Bahnemann D. Heterogeneous photocatalysed degradation of two selected pesticide derivatives, triclopyr and daminozid in aqueous suspensions of titanium dioxide. J Environ Manage, 2006, 80: 99–106
Sharma A K, Locke B R, Arce P, et al. A preliminary study of pulsed streamer corona discharge for the degradation of phenol in aqueous solutions. Hazard Waste Hazard, 1993, 10: 209–219
Joshi A A, Locke B R, Arce P, et al. Formation of hydroxyl radicals, hydrogen peroxide and aqueous electrons by pulsed streamer corona discharge in aqueous solution. J Hazard Mater, 1995, 41: 3–30
Willberg D M, Lang P S, Hochemer R H, et al. Degradation of 4-chlorophenol, 3,4-dichloroaniline and 2,4,6-trinitrotoluene in an electrohydraulic discharge reactor. Environ Sci Technol, 1996, 30: 2526–2534
Lang P S, Ching W K, Willberg D M, et al. Oxidative degradation of 2,4,6-Trinitrotoluene by ozone in an electrohydraulic discharge reactor. Environ Sci Technol, 1998, 32: 3142–3148
Sunka P, Babicky V, Clupek M, et al. Generation of chemically active species by electrical discharges in water. Plasma Sources Sci Technol, 1999, 8: 258–265
Hoeben W F L M, Veldhuizen E M, Rutgers W R, et al. The degradation of aqueous phenol solutions by pulsed positive corona discharges. Plasma Sources Sci Technol, 2000, 9: 361–369
Sun B, Sato M, Clements J S. Oxidative processes occurring when pulsed high voltage discharges degrade phenol in aqueous solution. Environ Sci Technol, 2000, 34: 509–513
Wen Y Z, Jiang X Z. Pulsed corona discharge-induced reaction of acetophenone in water. Plasma Chem Plasma Process, 2000, 21: 345–354
Chen Y S, Zhang X S, Dai Y C, et al. Pulsed high-voltage discharge plasma for degradation of phenol in aqueous solution. Sep Purif Technol, 2004, 34: 5–12
Sano N, Fujimoto T, Kawashima T, et al. Influence of dissolved inorganic additives on decomposition of phenol and acetic acid in water by direct contact of gas corona discharge. Sep Purif Technol, 2004, 37: 169–175
Locke B R, Sato M, Sunka P, et al. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind Eng Chem Res, 2006, 45: 882–905
Malik M A, Ubaid-ur-Rehman, Ghaffar A, et al. Synergistic effect of pulsed corona discharges and ozonation on decolourization of methylene blue in water. Plasma Sources Sci Technol, 2002, 11: 236–240
Gao J Z, Wang X Y, Hu Z G, et al. Plasma degradation of dyes in water with contact glow discharge electrolysis. Water Res, 2003, 37: 267–272
Sugiarto A T, Ito S, Ohshima T, et al. Oxidative decoloration of dyes by pulsed discharge plasma in water. J Electrost, 2003, 58: 135–145
Yang B, Lei L C, Zhou M H. Synergistic effects of liquid and gas phase discharges using pulsed high voltage for dyes degradation in the presence of oxygen. Chemosphere, 2005, 60: 405–411
Wang H J, Li J, Quan X. Decoloration of azo dye by a multi-needle-to-plate high-voltage pulsed corona discharge system in water. J Electrost, 2006, 64: 416–421
Clements J S, Sato M, Davis R H. Preliminary investigation of prebreakdown phenomena and chemical reactions using a pulsed high voltage discharge in water. IEEE Trans Ind Appl, 1987, IA-23: 224–235
Kuo S P, Bivolaru D. Electric discharge in the presence of supersonic shocks. Phys Lett A, 2003, 313: 101–105
Robinson J W, Ham M, Balater A N. Ultraviolet radiation from electronical discharges in water. J Appl Phys, 1973, 44: 72–76
Sun B, Sato M, Clements J S. Optical study of active species produced by a pulsed streamer corona discharge in water. J Electrost, 1997, 39: 189–202
Grymonpré D R, Finney W C, Clark R J, et al. Suspended activated carbon particles and ozone formation in aqueous-phase pulsed corona discharge reactors. Ind Eng Chem Res, 2003, 42: 5117–5134
Piskarev I M, Rylova A E, Sevast’yanov A I. Formation of ozone and hydrogen peroxide during an electrical discharge in the solution-gas system. Russ J Electrochem, 1996, 32: 827–829
Sato M, Ohgiyama T, Clements J S. Formation of chemical species and their effects on microorganisms using a pulsed high-voltage discharge in water. IEEE Trans Ind Appl, 1996, 32: 106–112
Ono R, Oda T. Measurement of hydroxyl radicals in pulsed corona discharge. J Electrost, 2002, 55: 333–342
Lukes P, Appleton A T, Locke B R. Hydrogen peroxide and ozone formation in hybrid gas-liquid electrical discharge reactors. IEEE Trans Ind Appl, 2004, 40: 60–67
Hao X L, Zhou M H, Zhang Y, et al. Enhanced degradation of organic pollutant 4-chlorophenol in water by non-thermal plasma process with TiO2. Plasma Chem Plasma Process, 2006, 26: 455–468
Zhang Y, Zhou M H, Hao X L, et al. Degradation mechanisms of 4-chlorophenol in a novel gas-liquid hybrid discharge reactor by pulsed high voltage system with oxygen or nitrogen bubbling. Chemosphere, 2007, 67: 702–711
Lei L C, Hao X L, Zhang Y, et al. Wastewater treatment using a heterogeneous magnetic (Fe3O4) non-thermal plasma process. Plasma Process Polym, 2007, 4: 455–462
Yan K, Hui H, Cui M, et al. Corona induced non-thermal plasmas: Fundamental study and industrial applications. J Electrost, 1998, 44: 17–39
Bessekhouad Y, Chaoui N, Trzpit M, et al. UV-vis versus visible degradation of Acid Orange II in a coupled CdS/TiO2 semiconductors suspension. J Photoch Photobio A, 2006, 183: 218–224
Xiong Y, Strunk P J, Xia H Y, et al. Treatment of dye wastewater containing acid orange II using a cell with three-phase three-dimensional electrode. Water Res, 2001, 35: 4226–4230
Kawashima N, Fujimoto N, Meguro K. Determination of critical micelle concentration of several nonionic surfactants by azo-hydrazone tautomerism of anionic dye. J Colloid Interf Sci, 1985, 103: 459–465
Yan K, van Heesch E J M, Pemen A J M, et al. A high-voltage pulse generator for corona plasma generation. IEEE Trans Ind Appl, 2002, 38: 866–872
Yan K, van Heesch E J M, van Veldhuizen E M, et al. Evaluation of pulsed power supply for producing non-thermal plasma by using positive pulsed streamer corona. J Adv Oxid Technol, 1999, 4: 312–318
Rea M, Yan K. Evaluation of pulse voltage generators. IEEE Trans Ind Appl, 1995, 31: 507–512
Yan K, van Heesch E J M, Pemen A J M, et al. From chemical kinetics to streamer corona reactor and voltage pulse generator. Plasma Chem Plasma Process, 2001, 21: 107–137
Liu C J, Zou J J, Yu K L, et al. Plasma application for more environmentally friendly catalyst preparation. Pure Appl Chem, 2006, 78: 1227–1238
Zou J J, Zhang Y P, Liu C J, et al. Hydrogen production from dimethyl ether using corona discharge plasma. J Power Sources, 2007, 163: 653–657
Zhang Q H, Barbosa-Cánovas G V, Swanson B G. Engineering aspects of pulsed electrical field pasteurization. J Food Eng, 1995, 25: 261–281
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (Grant Nos. 20336030, 90610005, 20576120 and U0633003), and Distinguished Youth Foundation of Zhejiang Province (Grant No. RC 02060)
About this article
Cite this article
Shen, Y., Lei, L. & Zhang, X. Evaluation of energy transfer and utilization efficiency of azo dye removal by different pulsed electrical discharge modes. Chin. Sci. Bull. 53, 1824–1834 (2008). https://doi.org/10.1007/s11434-008-0251-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-008-0251-3