Abstract
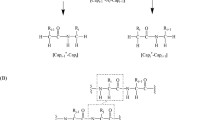
In order to get more reliable electronic structures of proteins in aqueous solution, it is necessary to construct a potential of water molecules for protein’s electronic structure calculation. The lysine is a hydrophilic amino acid. It is positively charged (Lys+) in neutral water solution. The first-principles, all-electron, ab initio calculations, based on the density functional theory, have been performed to construct such an equivalent potential of water molecules for lysine (Lys+). The process consists of three parts. First, the electronic structure of the cluster containing Lys+ and water molecules is calculated. By adjusting the positions of water molecules, the geometric structure of the cluster having minimum total energy is determined. Then, based on the structure, the electronic structure of Lys+ with the potential of water molecules is calculated using the self-consistent cluster-embedding (SCCE) method. Finally, the electronic structure of Lys+ with the potential of dipoles is calculated. The dipoles are adjusted so that the electronic structure of Lys+ with the potential of dipoles is close to that of water molecules. Thus the equivalent potential of water molecules for the electronic structure of lysine is obtained. The major effect of water molecules on lysine’s electronic structure is raising the occupied eigenvalues about 0.5032 eV, and broadening energy gap 89%. The effect of water molecules on the electronic structure of lysine can be simulated by dipoles potential.
Similar content being viewed by others
References
Zheng H P. One-electron approach and the theory of the self-consistent cluster-embedding calculation method. Phys Lett A, 1997, 226: 223–231, 453
Zheng H P. Self-consistent cluster-embedding calculation method and the calculated electronic structure of NiO. Phys Rev B, 1993, 48: 14868–14883
Zheng H P. Electronic structure of CoO. Physica B, 1995, 212: 125–138
Zheng H, Rao B K, Khanna S N, et al. Electronic structure and binding energies of hydrogen-decorated vacancies in Ni. Phys Rev B, 1997, 55: 4174–4181
Zheng H, Wang Y, Ma G. Electronic structure of LaNi5 and its hydride LaNi5H7. Eur Phys J B, 2002, 29: 61–69
He J, Zheng H P. The electronic structure of GaN and a single Ga-vacancy in GaN crystal. Acta Physica Sinica (in Chinese), 2002, 51: 2580–2588
Zheng H P. Electronic structure of trypsin inhibitor from squash seeds in aqueous solution. Phys Rev E, 2000, 62: 5500–5508
Zheng H P. First principle ab initio calculation of the electronic structure of protein molecule. Prog Phys (in Chinese), 2000, 20: 291–300
Zheng H P. Ab initio calculations of the electronic structures and biological functions of protein molecules. Mod Phys Lett B, 2002, 16: 1151–1162
Zheng H P. Electronic structures of Ascaris trypsin inhibitor in solution. Phys Rev E, 2003, 68: 051908
Lazaridis T, Karplus M. Discrimination of the native from misfolded protein models with an energy function including implicit solvation. J Mol Boil, 1999, 288(3): 477–487
Lazaridis T, Karplus M. Effective energy function for proteins in solution. Proteins: Struct Funct Genet, 1999, 35: 133–152
Klamt A, Schüürmann G COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. Chem Soc Perkin Trans, 1993, 2: 799–805
York D M, Karplus M. A smooth solvation potential based on the Conductor-Like Screening Model. J Phys Chem A, 1999, 103(50): 11060–11079
Guo H, Karplus M. Solvent influence on the stability of the peptide hydrogen bond: A supramolecular cooperative effect. J Phys Chem, 1994, 98: 7104–7105
Schaefer M, Karplus M. A comprehensive analytical treatment of continuum electrostatics. J Phys Chem, 1995, 100(5): 1578–1599
Ren C L, Hu Y D, Li D Q et al. A new model for the total interaction energy between two surfaces in aqueous solutions. J Adhes, 2004, 80: 831–849
Massova I, Kollman P A. Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to predict ligand binding. Persp Drug Disc Design, 2000, 18: 113–135
Wang D C. Protein Project (in Chinese). Beijing: Chemical Industry Press, 2002. 1–6
Chen H. Electronic structure of clusters: Applications to high-T c superconductors. Dissertation for the Doctoral Degree. Baton Rouge: Louisiana State University, 1988
Zheng H. Self-consistent cluster embedding calculation method and the electronic structure of NiO and CoO. Dissertation for the Doctoral Degree. Baton Rouge: Louisiana State University, 1993
Hohenberg P, Kohn W. Inhomogeneous electron gas. J Phys Rev B, 1964, 136: 864–871
Kohn W, Sham L J. Self-consistent equations including exchange and correlation effects. Phys Rev A, 1965, 140: 1133–1138
Chen H, Callaway J, Misra P K. Electronic structure of Cu-O chains in the high-T c superconductor Yba2Cu3O7. Phys Rev B, 1988, 38: 195–203
Chen H, Callaway J. Local electronic structure and magnetism of 3d transition-metal impurities (Cr, Mn, Fe, Co, and Ni) in La2−xSrxCuO4. Phys Rev B, 1991, 44: 2289–2296
Zheng H P, He J. Limitations of conventional one-electron approximation methods. J Tongji Univ (in Chinese), 2001, 29: 593–597
Xu W H, Zheng H P. Theoretic calculations of Co and Ni clusters with different sizes. J Tongji Univ (in Chinese), 2003, 31: 374–378
Lin S J, Zheng H P. Electronic structure of new oxygen molecule O4. J Tongji Univ (in Chinese), 2004, 32: 551–555
Hao J A, Zheng H P. Theoretical calculation of structures and properties of Ga6N6 cluster. Acta Physica Sinica (in Chinese), 2004, 53: 1044–1049
Zheng H P, Hao J A. Ab initio study of the electronic properties of the planar Ga5N5 cluster. Chin Phys, 2005, 14: 529–532
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 30470410) and the Science and Technology Development Foundation of Shanghai (Grant No. 03JC14070)
Rights and permissions
About this article
Cite this article
Li, C., Zheng, H. & Wang, X. The equivalent potential of water molecules for electronic structure of lysine. SCI CHINA SER G 50, 15–30 (2007). https://doi.org/10.1007/s11433-007-0003-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11433-007-0003-4