Abstract
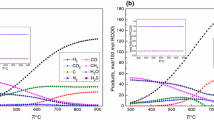
A two-dimensional transient model has been developed to describe the catalytic methane reforming (MSR) coupled with simultaneous CO2 removal by different absorbents under non-isothermal, non-isobaric and non-adiabatic operating conditions. The influences of temperature, pressure and steam/carbon (S/C) on enhancement were taken into account. The results showed that the hydrogen mole fraction (dry basis) higher than 94% could be achieved using Li4SiO4, CaO, and HTC as CO2 acceptors at the operating conditions of 550°C and 0.1 MPa. When the reaction temperature varied from 500°C to 600°C, the initial CO2 capture rates were HTC>CaO>Li4SiO4>Li2ZrO3, and the saturation rates HTC>CaO>Li4SiO4>Li2ZrO3. Increasing the reaction temperature would improve the CO2 capture rate and available CO2 capacity. For Li4SiO4, although the adsorbing rate increased as the operating temperature increased, the capacity almost did not change. At 550°C, increasing the working pressure could promote the enhancing factors of Li4SiO4,Li2ZrO3 and HTC. There was an optimal steam/carbon ratio between 2–4.5 such that all CaO, Li4SiO4, HTC and Li2ZrO3 would obtain the biggest enhancement for H2 production at the pre-breakthrough stage.
Similar content being viewed by others
References
Jin H G, Zhang X L, Gao L, et al. Fundamental study of CO2 control technologies and policies in China. Sci China Ser E-Tech Sci, 2008, 38(9): 1495–1506
Kothari R, Buddhi D, Sawhney R L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew Sust Energ Rev, 2008, 12(2): 553–563
Hufton J R, Mayorga S, Sircar S. Sorption-enhanced reaction process for hydrogen production. A I Ch E J, 1999, 45(2): 248–256
Harrison P. Sorption-enhanced hydrogen production: A review. Ind Eng Chem Res, 2008, 47(17): 6486–6501
Lee D K, Baek I H, Yoon W L. Modeling and simulation for the methane steam reforming enhanced by in situ CO2 removal utilizing the CaO carbonation for H2 production. Chem Eng Sci, 2004, 59(4): 931–942
Ochoa-Fernández E, Rusten H K, Jakobsen H A, et al. Sorption enhanced hydrogen production by steam methane reforming using Li2ZrO3 as sorbent: Sorption kinetics and reactor simulation. Catal Today, 2005, 106(1–4): 41–46
Rusten H K, Ochoa-Fernández E, Chen D, et al. Numerical Investigation of sorption enhanced steam methane reforming using Li2ZrO3 as CO2-acceptor. Ind Eng Chem Res, 2007, 46(13): 4435–4443
Rusten H K, Ochoa-Ferández E, Lindborg H, et al. Hydrogen production by sorption-enhanced steam methane reforming using Lithium Oxides as CO{on2}-acceptor. Ind Eng Chem Res, 2007, 46(25): 8729–8737
Venegas M J, Fregoso-Israel E, Escamilla R, et al. Kinetic and reaction mechanism of CO2 sorption on Li4SiO4: Study of the particle size effect. Ind Eng Chem Res, 2007, 46(25): 2407–2412
Ding Y, Alpay E. Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem Eng Sci, 2000, 55(17): 3461–3474
Pedernera M N, Piña J, Borio D O, et al. Use of a heterogeneous two-dimensional model to improve the primary steam reformer performance. Chem Eng J, 2003, 94(1): 29–40
Zahedi N M, Rowshanzamir S, Eikani M H. Autothermal reforming of methane to synthesis gas: Modeling and simulation. Int J Hydrogen Energy, 2009, 34(3): 1292–1300
Kumar S, Jitendra K. Hydrogen production by partial oxidation of methane: Modeling and simulation. Int J Hydrogen Energy, 2009, 34(16): 6655–6668
Groote A M D, Froment G F. Simulation of the catalytic partial oxidation of methane to synthesis gas. Appl Catal, A-General, 1996, 138(2): 245–264
Xu J, Froment G F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. A I Ch E J, 1989, 35(1): 88–96
Li C H, Finlayson B A. Heat transfer in packedbeds-A reevaluation. Chem Eng Sci, 1977, 32(9): 1055–1066
De W A P, Froment G F. Heat transfer in packed bed. Chem Eng Sci, 1972, 27(3): 567–576
Punčochář M, Drahoš J. The tortuosity concept in fixed and fluidized bed. Chem Eng Sci, 1993, 48(11): 2173–2175
Lee D K. An apparent kinetic model for the carbonation of calcium oxide by carbon dioxide. Chem Eng J, 2004, 100(1–3): 71–77
Reijers H T J, Boon J, Elzinga G D, et al. Modeling study of the sorption-enhanced reaction process for CO2 capture. I. Model development and validation. Ind Eng Chem Res, 2009, 48: 6966–6974
Barelli L, Bidini G, Gallorini F, et al. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy, 2008, 33(4): 554–570
Kenji E, Takehiko M, Masahiro K. Effect of equilibrium shift by using lithium silicate pellets in methane steam reforming. Int J Hydrogen Energy, 2008, 33(17): 4555–4559
Reijers H T J, Boon J, Elzinga G D, et al. Modeling study of the sorption-enhanced reaction process for CO2 capture: II. Application to steam-methane reforming. Ind Eng Chem Res, 2009, 48(15): 6975–6982
Ding Y, Alpay E. Adsorption-enhanced steam-methane reforming. Chem Eng Sci, 2000, 55: 3929–3940
Fauth D J, Frommell E A, Hoffman J S, et al. Eutectic salt promoted lithium zirconate: Novel high temperature sorbent for CO2 capture. Fuel Process Tech, 2005, 86(14–15): 1503–1521
Xiong R T, Ida J, Lin Y S. Kinetics of carbon dioxide sorption on potassium-doped lithium zirconate. Chem Eng Sci, 2003, 58(19): 4377–4385
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Zhao, Y., Zhang, J. et al. Hydrogen production through CO2 sorption-enhanced methane steam reforming: Comparison between different adsorbents. Sci. China Technol. Sci. 54, 2999–3008 (2011). https://doi.org/10.1007/s11431-011-4587-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-011-4587-6