Abstract
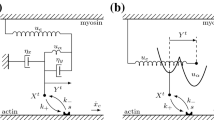
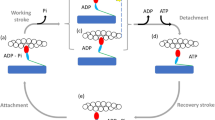
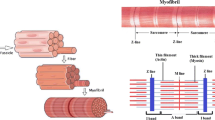
A non-equilibrium statistical method is used to study the collective characteristics of myosin II motors in a sarcomere during its contraction. By means of Fokker-Planck equation of molecular motors, we present a dynamic mechanical model for the sarcomere in skeletal muscle. This model has been solved with a numerical algorithm based on experimental chemical transition rates. The influences of ATP concentration and load on probability density, contraction velocity and maximum active force are discussed respectively. It is shown that contraction velocity and maximum isometric active force increase with the increasing ATP concentration and become constant when the ATP concentration reaches equilibrium saturation. Contraction velocity reduces gradually as the load force increases. We also find that active force begins to increase then decrease with the increasing length of sarcomere, and has a maximum value at the optimal length that all myosin motors can attach to actin filament. Our results are in good agreement with the Hill muscle model.
Similar content being viewed by others
References
Guo Z, Yin Y H. Coupling mechanism of multi-force interactions in the myosin molecular motor. Chin Sci Bull, 2010, 55(31): 3538–3544
Van Delden R A, Ter Wiel M K J, Pollard M M, et al. Unidirectional molecular motor on a gold surface. Nature, 2005, 437: 1337–1340
Guo Z, Yin Y H. Casimir effect on adhesion interaction between myosin molecular motor and actin filament. Int J Nanosyst, 2010, 3: 9–15
Ren Q, Zhao Y P, Yue J C, et al. Biological application of multi-component nanowires in hybrid devices powered by F1-ATPase motors. Biomed Microdevices, 2006, 8: 201–208
Gao L, Liu Q, Zhang Y Y, et al. Constructing an array of anchored single-molecule rotors on gold surfaces. Phys Rev Lett, 2008, 101: 197209
Qi W, Duan L, Wang K W, et al. Motor protein CF0F1 reconstituted in lipid-coated hemoglobin microcapsules for ATP synthesis. Adv Mater, 2008, 20: 601–605
Vermeulen K C, Stienen G J M, Schmidt C F. Cooperative behavior of molecular motors. J Muscle Res Cell Motil, 2002, 23: 71–79
Huxley A F, Niedergerke R. Structural changes in muscle during contraction. Nature, 1954, 173: 971–973
Julicher F, Ajdari A, Prost J. Modeling molecular motors. Rev Mod Phys, 1997, 69: 1269–1281
Shu Y G, Shi H L. Cooperative effects on the kinetics of ATP hydrolysis in collective molecular motors. Phys Rev E, 2004, 69: 021912
Duke T A J. Molecular model of muscle contraction. Proc Natl Acad Sci USA, 1999, 96: 2770–2775
Lan G, Sun S X. Dynamics of myosin-driven skeletal muscle contraction: I. Steady-state force generation. Biophys J, 2005, 88: 4107–4117
Lecarpentier Y, Blanc F X, Quillard J, et al. Statistical mechanics of myosin molecular motors in skeletal muscles. J Theor Biol, 2005, 235: 381–392
Rayment I, Holden H M, Whittaker M, et al. Structure of the actin-myosin complex and its implications for muscle contraction. Science, 1993, 261: 56–65
Xing J, Wang H, Oster G. From continuum Fokker-Planck models to discrete kinetic models. Biophys J, 2005, 89: 1551–1563
Wang H, Peskin C S, Elston T C. A robust numerical algorithm for studying biomolecular transport processes. J Theor Biol, 2003, 221: 491–511
Veigel C, Molloy J E, Schmitz S, et al. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat cell biol, 2003, 5: 980–986
David K, Carlos B. The mechanochemistry of molecular motors. Biophys J, 2000, 78: 541–556
Shu Y G, Yue J C, Ou-Yang Z C. F0F1-ATPase, rotary motor and biosensor. Nanoscale, 2010, 2: 1284–1293
Reimann P. Brownian motors: Noisy transport far from equilibrium. Phys Rep, 2002, 361: 57–265
Finer J T, Simmons R M, Spudich J A. Single myosin molecule mechanics: Pico Newton forces and nano metre steps. Nature, 1994, 368: 113–119
Shimamoto Y, Kono F, Suzuki M, et al. Nonlinear force-length relationship in the ADP-induced contraction of skeletal myofibrils. Biophys J, 2007, 93: 4330–4341
Hill A V. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B, 1938, 126: 136–195
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, Y., Guo, Z. Collective mechanism of molecular motors and a dynamic mechanical model for sarcomere. Sci. China Technol. Sci. 54, 2130–2137 (2011). https://doi.org/10.1007/s11431-011-4458-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-011-4458-1