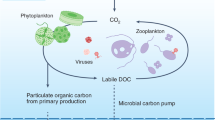
Abstract
In nature, there are two conformational types of amino acids: L- and D-isomers. The L-amino acids are the predominant form and are used mainly for protein synthesis, while the D-amino acids are few in quantity but more diverse in terms of their biological functions. D-amino acids are produced by many marine microbes, which are important players in carbon and energy cycles in the ocean. As the major constituent of the marine organic carbon pool, D-amino acids can persist in the water column for a long time before being further transformed by chemical or biological processes or transported through physical processes (such as absorption and aggregation). This article reviews the microbial synthesis of D-amino acids, their physiological function and metabolism in microbes, and the contribution of D-amino acids as a carbon source to the oceanic carbon reservoir.
Similar content being viewed by others
References
Abe H, Okuma E, Amano H, Noda H, Watanabe K. 1999. Role of free d- and l-alanine in the Japanese mitten crab Eriocheir japonicus to intracellular osmoregulation during downstream spawning migration. Comp Biochem Phys A, 123: 55–59
Auclair J, Patton R. 1950. On the occurrence of D-alanine in the haemolymph of the milkweed bug, oncopeltus fasciatus. Rev Can Biol, 9: 3
Azam F, Malfatti F. 2007. Microbial structuring of marine ecosystems. Nature Rev Microbiol, 5: 782–791
Barja I, Núñez L. 1999. Microcalorimetric measurements of the influence of glucose concentration on microbial activity in soils. Soil Biol Biochem, 31: 441–447
Brodowski S, Amelung W, Lobe I, Du Preez C C. 2005. Losses and biogeochemical cycling of soil organic nitrogen with prolonged arable cropping in the South African Highveld—Evidence from D- and L-amino acids. Biogeochemistry, 71: 17–42
Brown M, Lauro F, Demaere M, Muir L, Wilkins D, Thomas T, Riddle M, Fuhrman J, Andrews-Pfannkoch C, Hoffman J. 2012. Global biogeography of SAR11 marine bacteria. Mol Syst Biol, 8: 595
Brückner H, Westhauser T. 2003. Chromatographic determination of L-and D-amino acids in plants. Amino Acids, 24: 43–55
Dauwe B, Middelburg J J. 1998. Amino acids and hexosamines as indicators of organic matter degradation state in North Sea sediments. Limnol Oceanogr, 43: 782–798
Delfosse V, Girard E, Birck C, Delmarcelle M, Delarue M, Poch O, Schultz P, Mayer C. 2009. Structure of the archaeal pab87 peptidase reveals a novel self-compartmentalizing protease family. PLoS One, 4: e4712
DeLong E F. 1992. Archaea in coastal marine environments. Proc Natl Acad Sci USA, 89: 5685–5689
Eichinger M, Poggiale J C, Van Wambeke F, Lefevre D, Sempere R. 2006. Modelling DOC assimilation and bacterial growth efficiency in biodegradation experiments: A case study in the Northeast Atlantic Ocean. Aquat Microbial Ecol, 43: 139–151
Fernandes L, Garg A, Borole D V. 2014. Amino acid biogeochemistry and bacterial contribution to sediment organic matter along the western margin of the Bay of Bengal. Deep-Sea Res Part I: Oceanogr Res Pap, 83: 81–92
Flemming H C, Wingender J. 2010. The biofilm matrix. Nature Rev Microbiol, 8: 623–633
Forsum O, Svennerstam H, Ganeteg U, Näsholm T. 2008. Capacities and constraints of amino acid utilization in Arabidopsis. New Phytol, 179: 1058–1069
Gehlen M. 2006. Reconciling surface ocean productivity, export fluxes and sediment composition in a global biogeochemical ocean model. Biogeosciences, 3: 521–537
Giovannoni S, Rappé M. 2000. Evolution, diversity, and molecular ecology of marine prokaryotes. In: Kirchman D, ed. Microbial Ecology of the Oceans. New York: Wiley. 47–84
Gördes D, Kolukisaoglu Ü, Thurow K. 2011. Uptake and conversion of D-amino acids in Arabidopsis thaliana. Amino Acids, 40: 553–563
Halvorson H O, Spiegelman S. 1952. The inhibition of enzyme formation by amino acid analogues. J Bacteriol, 64: 207–221
Herndl G J, Reinthaler T, Teira E, Aken H M V, Veth C, Pernthaler A, Pernthaler J. 2005. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl Environ Microbiol, 72: 2303–2309
Hertkorn N, Benner R, Frommberger M, Schmitt-Kopplin P, Witt M, Kaiser K, Kettrup A, Hedges J I. 2006. Characterization of a major refractory component of marine dissolved organic matter. Geochim Cosmochim Acta, 70: 2990–3010
Hill P W, Quilliam R S, DeLuca T H, Farrar J, Farrell M, Roberts P, Newsham K K, Hopkins D W, Bardgett R D, Jones D L. 2011. Acquisition and assimilation of nitrogen as peptide-bound and D-enantiomers of amino acids by wheat. PLoS One, 6: e19220
Hills G. 1949. Chemical factors in the germination of spore-bearing aerobes. The effects of amino-acids on the germination of Bacillus anthracis, with some observations on the relation of optical form to biological activity. Biochem J, 45: 363
Hochbaum A I, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. 2011. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J Bacteriol, 193: 5616–5622
Huang Y, Nishikawa T, Satoh K, Iwata T, Fukushima T, Homma H, Imai K. 1998. Urinary excretion of D-serine in human: Comparison of different ages and species. Biol Pharm Bull, 21: 156
Jensen P, Fenical W. 1995. The relative abundance and seawater requirements of Gram-positive bacteria in near-shore tropical marine samples. Microb Ecol, 29: 249–257
Jiao N, Herndl G J, Hansell D A, Benner R, Kattner G, Wilhelm S W, Kirchman D L, Weinbauer M G, Luo T, Chen F, Azam F. 2010. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nature Rev Microbiol, 8: 593–599
Jørgensen N O G, Stepanaukas R, Pedersen A G U, Hansen M, Nybroe O. 2003. Occurrence and degradation of peptidoglycan in aquatic environments. FEMS Microbiol Ecol, 46: 269–280
Jørgensen N O G, Middelboe M. 2006. Occurrence and bacterial cycling of D-amino acid isomers in an estuarine environment. Biogeochemistry, 81: 77–94
Kaiser K, Benner R. 2008. Major bacterial contribution to the ocean reservoir of detrital organic carbon and nitrogen. Limnol Oceanogr, 53: 99–112
Kandler O, König H. 1978. Chemical composition of the peptidoglycanfree cell walls of methanogenic bacteria. Arch Microbiol, 118: 141–152
Kandler O, König H. 1998. Cell wall polymers in Archaea (Archaebacteria). Cell Mol Life Sci, 54: 305–308
Karatan E, Watnick P. 2009. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev, 73: 310–347
Karner M B, DeLong E F, Karl D M. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature, 409: 507–510
Kawai Y, Ishii Y, Arakawa K, Uemura K, Saitoh B, Nishimura J, Kitazawa H, Yamazaki Y, Tateno Y, Itoh T. 2004. Structural and functional differences in two cyclic bacteriocins with the same sequences produced by lactobacilli. Appl Environ Microbiol, 70: 2906–2911
Kawasaki N, Benner R. 2006. Bacterial release of dissolved organic matter during cell growth and decline: Molecular origin and composition. Limnol Oceanogr, 51: 2170–2180
Kim P M, Duan X, Huang A S, Liu C Y, Ming G L, Song H, Snyder S H. 2010. Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci USA, 107: 3175–3179
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. 2010. D-amino acids trigger biofilm disassembly. Science, 328: 627–629
Könneke M, Bernhard A E, José R, Walker C B, Waterbury J B, Stahl D A. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature, 437: 543–546
Lam H, Oh D C, Cava F, Takacs C N, Clardy J, de Pedro M A, Waldor M K. 2009. D-Amino acids govern stationary phase cell wall remodeling in bacteria. Science, 325: 1552–1555
Lee C, Bada J L. 1977. Dissolved amino acids in the equatorial Pacific, the Sargasso Sea, and Biscayne Bay. Limnol Oceanogr, 22: 502–510
Li C, Yao X, Lu C D. 2009. Regulation of the dauBAR operon and characterization of D-amino acid dehydrogenase DauA in arginine and lysine catabolism of Pseudomonas aeruginosa PAO1. Microbiology, 156: 60–71
Lomstein B A, Jorgensen B B, Schubert C J, Niggemann J. 2006. Amino acid biogeo- and stereochemistry in coastal Chilean sediments. Geochim Cosmochim Acta, 70: 2970–2989
Matsumoto M, Homma H, Long Z, Imai K, Iida T, Maruyama T, Aikawa Y, Endo I, Yohda M. 1999. Occurrence of free D-amino acids and aspartate racemases in hyperthermophilic Archaea. J Bacteriol, 181: 6560–6563
McCarthy M D, Hedges J I, Benner R. 1998. Major bacterial contribution to marine dissolved organic nitrogen. Science, 281: 231–234
Miyoshi Y, Konno R, Sasabe J, Ueno K, Tojo Y, Mita M, Aiso S, Hamase K. 2012. Alteration of intrinsic amounts of D-serine in the mice lacking serine racemase and D-amino acid oxidase. Amino acids, 43: 1919–1931
Moriarty D, Hayward A. 1982. Ultrastructure of bacteria and the proportion of Gram-negative bacteria in marine sediments. Microb Ecol, 8: 1–14
Morikawa M, Daido H, Takao T, Murata S, Shimonishi Y, Imanaka T. 1993. A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J Bacteriol, 175: 6459–6466
Nagata T, Meon B, Kirchman D. 2003. Microbial degradation of peptidoglycan in seawater. Limnol Oceanogr, 48: 745–754
Nagata Y, Tanaka K, Iida T, Kera Y, Yamada R H, Nakajima Y, Fujiwara T, Fukumori Y, Yamanaka T, Koga Y. 1999. Occurrence of D-amino acids in a few archaea and dehydrogenase activities in hyperthermophile Pyrobaculum islandicum. Biochim Biophys Acta, 1435: 160–166
Ogawa H, Tanoue E. 2003. Dissolved organic matter in oceanic waters. J Oceanogr, 59: 129–147
Ohnishi M, Saito M, Wakabayashi S, Ishizuka M, Nishimura K, Nagata Y, Kasai S. 2008. Purification and characterization of serine racemase from a hyperthermophilic archaeon, Pyrobaculum islandicum. J Bacteriol, 190: 1359–1365
Pedersen A-GU, Thomsen T R, Lomstein B A, Jørgensen N O G. 2001. Bacterial influence on amino acid enantiomerization in a coastal marine sediment. Limnol Oceanogr, 46: 1358–1369
Ravenschlag K, Sahm K, Amann R. 2001. Quantitative molecular analysis of the microbial community in marine Arctic sediments (Svalbard). Appl Environ Microbiol, 67: 387–395
Reeburgh W S. 1997. Figures summarizing the global cycles of biogeochemically important elements. Bull Ecol Soc Am, 78: 260–267
Rydon H. 1947. D-amino acids in microbiological chemistry. Biochem J, 41: xxxvi
Schleifer K H, Kandler O. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev, 36: 407
Snyder S H, Kim P M. 2000. D-amino acids as putative neurotransmitters: Focus on D-serine. Neurochem Res, 25: 553–560
Vollmer W, Blanot D, De Pedro M A. 2008. Peptidoglycan structure and architecture. FEMS Microbiol Rev, 32: 149–167
Xu H J, Liu Y. 2011. Reduced microbial attachment by D-amino acid-inhibited AI-2 and EPS production. Water Res, 45: 5796–5804
Yokoyama T, Kan-no N, Ogata T, Kotaki Y, Sato M, Nagahisa E. 2003. Presence of free D-amino acids in microalgae. Biosci Biotechnol Biochem, 67: 388
Yoshimura T, Esak N. 2003. Amino acid racemases: Functions and mechanisms. J Biosci Bioeng, 96: 103–109
Zhang G, Sun H J. 2014. Racemization in reverse: Evidence that D-amino acid toxicity on earth is controlled by bacteria with racemases. PLoS One, 9: e92101
Zhang Y, Sintes E, Chen J, Dai M, Jiao N, Herndl G J. 2009. Role of mesoscale cyclonic eddies in the distribution and activity of Archaea and Bacteria in the South China Sea. Aquat Microb Ecol, 56: 65–79
Zhang Z, Li Z, Jiao N. 2014. Effect of D-amino acids of the EPS production and cell aggregation of Alteromonas macleodii stain JL2069. Curr Microbiol, 68: 751–755
Zhuang R, Chen H, Yao J, Li Z, Burnet J E, Choi M M F. 2011. Impact of beta-cypermethrin on soil microbial community associated with its bioavailability: A combined study by isothermal microcalorimetry and enzyme assay techniques. J Hazard Mater, 189: 323–328
Zobell C E. 1946. Marine Microbiology, A monograph on Hydrobacteriology. Waltham: Chronica Botanica Press
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Zheng, Q. & Jiao, N. Microbial D-amino acids and marine carbon storage. Sci. China Earth Sci. 59, 17–24 (2016). https://doi.org/10.1007/s11430-015-5155-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11430-015-5155-x