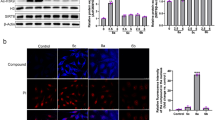
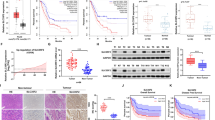
Abstract
Despite the use of many types of chemotherapies for pancreatic cancer, optimal efficacy has not been obtained so far. Pancreatic cancer shows a high incidence of TP53 mutations, inactivating its tumor suppressor activity. In this study, we identified sodium cantharidinate as a novel, potential anti-pancreatic cancer agent that activates p53 function. Sodium cantharidinate reduced the viability of pancreatic cancer cells, including the human primary pancreatic cancer cells, PANC-1, AsPC-1, SW1990 and BXPC-3, in a dose-dependent manner. Sodium cantharidinate induced apoptosis and DNA damage of pancreatic cancer cells. Furthermore, proteome-wide sequencing analysis detected a marked perturbation in p53 signaling pathway on PANC-1 cells upon sodium cantharidinate. Consistent with the previous results, sodium cantharidinate treatment decreased Bcl-2 and mitochondrial cytochrome-c protein expression, as well as phosphorylation of MDM2; meanwhile, it increased the levels of cleaved-caspase-3, cleaved-caspase-9, cleaved-PARP, Bax, and phosphorylated p53, thus inducing the apoptosis of pancreatic cancer cells. The p53-activating effect of sodium cantharidinate was strongly abrogated by treatment with TP53-targeting shRNA. Moreover, sodium cantharidinate inhibited neoplasm growth via the JAK2-STAT3 pathway, which was inhibited by shRNA-TP53 and triggered by combination with gemcitabine. Combination therapy indicated that sodium cantharidinate and gemcitabine synergistically reduced ex vivo and in vivo growth of pancreatic neoplasm. Further docking studies revealed the different binding fates of sodium cantharidinate to activate wild-type p53 function. Thus, sodium cantharidinate could be a potential agent with promising anti-pancreatic cancer efficacy.
Similar content being viewed by others
References
Agarwal, C., Tyagi, A., Kaur, M., and Agarwal, R. (2007). Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis 28, 1463–1470.
Aubrey, B.J., Kelly, G.L., Janic, A., Herold, M.J., and Strasser, A. (2018). How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ 25, 104–113.
Burris, H.A., Moore, M.J., Andersen, J., Green, M.R., Rothenberg, M.L., Modiano, M.R., Cripps, M.C., Portenoy, R.K., Storniolo, A.M., Tarassoff, P., et al. (1997). Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15, 2403–2413.
Chen, L., Zhou, D., Liu, Z., Huang, X., Liu, Q., Kang, Y., Chen, Z., Guo, Y., Zhu, H., and Sun, C. (2018). Combination of gemcitabine and erlotinib inhibits recurrent pancreatic cancer growth in mice via the JAK-STAT pathway. Oncol Rep https://doi.org/10.3892/or.2018.6198.
Chen, M., Hough, A.M., and Lawrence, T.S. (2000). The role of p53 in gemcitabine-mediated cytotoxicity and radiosensitization. Cancer Chemother Pharmacol 45, 369–374.
Chou, T.C. (2010). Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70, 440–446.
Deng, Y.Y., Zhang, W., Li, N. P., Lei, X.P., Gong, X.Y., Zhang, D.M., Wang, L., and Ye, W.C. (2017). Cantharidin derivatives from the medicinal insect Mylabris phalerata. Tetrahedron 73, 5932–5939.
Du, Y., Liu, Z., You, L., Hou, P., Ren, X., Jiao, T., Zhao, W., Li, Z., Shu, H., Liu, C., et al. (2017). Pancreatic cancer progression relies upon mutant p53-induced oncogenic signaling mediated by NOP14. Cancer Res 77, 2661–2673.
Esser, C., Scheffner, M., and Höhfeld, J. (2005). The chaperone-associated ubiquitin ligase CHIP is able to target p53 for proteasomal degradation. J Biol Chem 280, 27443–27448.
Fiorini, C., Cordani, M., Padroni, C., Blandino, G., Di Agostino, S., and Donadelli, M. (2015). Mutant p53 stimulates chemoresistance of pancreatic adenocarcinoma cells to gemcitabine. Biochim Biophys Acta Mol Cell Res 1853, 89–100.
Freed-Pastor, W.A., and Prives, C. (2012). Mutant p53: one name, many proteins. Genes Dev 26, 1268–1286.
Fridman, J.S., and Lowe, S.W. (2003). Control of apoptosis by p53. Oncogene 22, 9030–9040.
Han, L., Ren, C., Li, L., Li, X., Ge, J., Wang, H., Miao, Y.L., Guo, X., Moley, K.H., Shu, W., et al. (2018). Embryonic defects induced by maternal obesity in mice derive from Stella insufficiency in oocytes. Nat Genet 50, 432–442.
Hartwig, W., Werner, J., Jäger, D., Debus, J., and Büchler, M.W. (2013). Improvement of surgical results for pancreatic cancer. Lancet Oncol 14, e476–e485.
Haupt, Y., Maya, R., Kazaz, A., and Oren, M. (1997). Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299.
Hill, R., Rabb, M., Madureira, P.A., Clements, D., Gujar, S.A., Waisman, D.M., Giacomantonio, C.A., and Lee, P.W.K. (2013). Gemcitabine-mediated tumour regression and p53-dependent gene expression: implications for colon and pancreatic cancer therapy. Cell Death Dis 4, e791.
Huang, G., Yan, H., Ye, S., Tong, C., and Ying, Q.L. (2014). STAT3 phosphorylation at tyrosine 705 and serine 727 differentially regulates mouse ESC fates. Stem Cells 32, 1149–1160.
Kandoth, C., McLellan, M.D., Vandin, F., Ye, K., Niu, B., Lu, C., Xie, M., Zhang, Q., McMichael, J.F., Wyczalkowski, M.A., et al. (2013). Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339.
Kubbutat, M.H.G., Jones, S.N., and Vousden, K.H. (1997). Regulation of p53 stability by Mdm2. Nature 387, 299–303.
Lang, G.A., Iwakuma, T., Suh, Y.A., Liu, G., Rao, V.A., Parant, J.M., Valentin-Vega, Y.A., Terzian, T., Caldwell, L.C., Strong, L.C., et al. (2004). Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872.
Lepage, C., Capocaccia, R., Hackl, M., Lemmens, V., Molina, E., Pierannunzio, D., Sant, M., Trama, A., Faivre, J., Hackl, M., et al. (2015). Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: Results of EUROCARE-5. Eur J Cancer 51, 2169–2178.
Li, J., Yang, L., Gaur, S., Zhang, K., Wu, X., Yuan, Y.C., Li, H., Hu, S., Weng, Y., and Yen, Y. (2014). Mutants TP53 p.R273H and p.R273C but not p.R273G enhance cancer cell malignancy. Hum Mutat 35, 575–584.
Li, W., Xie, L., Chen, Z., Zhu, Y., Sun, Y., Miao, Y., Xu, Z., and Han, X. (2010). Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci 101, 1226–1233.
Liu, M., Xu, C., and Sun, Y. (2019). Efficacy and safety of sodium cantharidinate and vitamin B6 injection for the treatment of digestive system neoplasms: a meta-analysis of randomized controlled trials. Drug Des Devel Ther Volume 13, 183–203.
Llambi, F., Wang, Y.M., Victor, B., Yang, M., Schneider, D.M., Gingras, S., Parsons, M.J., Zheng, J.H., Brown, S.A., Pelletier, S., et al. (2016). BOK is a non-canonical BCL-2 family effector of apoptosis regulated by ER-associated degradation. Cell 165, 421–433.
Lukashchuk, N., and Vousden, K.H. (2007). Ubiquitination and degradation of mutant p53. Mol Cell Biol 27, 8284–8295.
Muller, P.A.J., and Vousden, K.H. (2014). Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 25, 304–317.
Niu, G., Wright, K.L., Ma, Y., Wright, G.M., Huang, M., Irby, R., Briggs, J., Karras, J., Cress, W.D., Pardoll, D., et al. (2005). Role of Stat3 in regulating p53 expression and function. Mol Cell Biol 25, 7432–7440.
Shao, H., Hong, G., and Luo, X. (2014). Evaluation of sodium cantharidinate/vitamin B6 in the treatment of primary liver cancer. J Can Res Ther 10, 75.
Shieh, S.Y., Ikeda, M., Taya, Y., and Prives, C. (1997). DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334.
Tao, R., Sun, W.Y., Yu, D.H., Qiu, W., Yan, W.Q., Ding, Y.H., Wang, G.Y., and Li, H.J. (2017). Sodium cantharidinate induces HepG2 cell apoptosis through LC3 autophagy pathway. Oncol Rep 38, 1233–1239.
Torre, L.A., Bray, F., Siegel, R.L., Ferlay, J., Lortet-Tieulent, J., and Jemal, A. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108.
Wang, J., Shi, Z.Q., Zhang, M., Xin, G.Z., Pang, T., Zhou, P., Chen, J., Qi, L.W., Yang, H., Xu, X., et al. (2015). Camptothecin and its analogs reduce amyloid-β production and amyloid-β42-induced IL-1β production. J Alzheimers Dis 43, 465–477.
Wang, Y., Suh, Y.A., Fuller, M.Y., Jackson, J.G., Xiong, S., Terzian, T., Quintás-Cardama, A., Bankson, J.A., El-Naggar, A.K., and Lozano, G. (2011). Restoring expression of wild-type p53 suppresses tumor growth but does not cause tumor regression in mice with a p53 missense mutation. J Clin Invest 121, 893–904.
Wörmann, S.M., Song, L., Ai, J., Diakopoulos, K.N., Kurkowski, M.U., Görgülü, K., Ruess, D., Campbell, A., Doglioni, C., Jodrell, D., et al. (2016). Loss of P53 function activates JAK2-STAT3 signaling to promote pancreatic tumor growth, stroma modification, and gemcitabine resistance in mice and is associated with patient survival. Gastroenterology 151, 180–193.e12.
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFC1711000 to P.L.), the National Natural Science Foundation of China (81772566 to J.L.), and in part by the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZCX201820 to X.X.), and the “Double First-Class” University Project (CPU2018GF04 to X.X.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Compliance and ethics
The author(s) declare that they have no conflict of interest.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, L., Thu, P.M. et al. Sodium cantharidinate, a novel anti-pancreatic cancer agent that activates functional p53. Sci. China Life Sci. 64, 1295–1310 (2021). https://doi.org/10.1007/s11427-019-1753-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-1753-3