Abstract
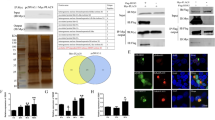
Interplay between the host and influenza virus has a pivotal role for the outcome of infection. The matrix proteins M2/BM2 from influenza (A and B) viruses are small type III integral membrane proteins with a single transmembrane domain, a short amino-terminal ectodomain and a long carboxy-terminal cytoplasmic domain. They function as proton channels, mainly forming a membrane-spanning pore through the transmembrane domain tetramer, and are essential for virus assembly and release of the viral genetic materials in the endosomal fusion process. However, little is known about the host factors which interact with M2/BM2 proteins and the functions of the long cytoplasmic domain are currently unknown. Starting with yeast two-hybrid screening and applying a series of experiments we identified that the β1 subunit of the host Na+/K+-ATPase β1 subunit (ATP1B1) interacts with the cytoplasmic domain of both the M2 and BM2 proteins. A stable ATP1B1 knockdown MDCK cell line was established and we showed that the ATP1B1 knockdown suppressed influenza virus A/WSN/33 replication, implying that the interaction is crucial for influenza virus replication in the host cell. We propose that influenza virus M2/BM2 cytoplasmic domain has an important role in the virus-host interplay and facilitates virus replication.
Similar content being viewed by others
References
Gao G F, Sun Y P. It is not just AIV: From avian to swine-origin influenza virus. Sci China Life Sci, 2010, 53:151–153 10.1007/s11427-010-0017-4, 20596968
Kang S, Yang I S, Lee J Y, et al. Epidemiologic study of human influenza virus infection in South Korea from 1999 to 2007: origin and evolution of A/Fujian/411/2002-like strains. J Clin Microbiol, 2010, 48:2177–2185 1:CAS:528:DC%2BC3cXpsVGntL4%3D, 10.1128/JCM.00209-10, 20392920
Khanna M, Gupta N, Gupta A, et al. Influenza A (H1N1) 2009: a pandemic alarm. J Biosci, 2009, 34:481–489 10.1007/s12038-009-0053-z, 19805908
Zhang W, Qi J X, Shi Y, et al. Crystal structure of the swine-origin A (H1N1)—2009 influenza A virus hemagglutinin (HA) reveals similar antigenicity to that of the 1918 pandemic virus. Protein & Cell, 2010, 1:459–467 1:CAS:528:DC%2BC3cXosleluro%3D, 10.1007/s13238-010-0059-1
Liu D, Liu X L, Yan J H, et al. Interspecies transmission and host restriction of avian H5N1 influenza virus. Sci China Ser C-Life Sci, 2009, 52:428–438 10.1007/s11427-009-0062-z
Knipe D M, Howley P M. Fields Virology. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer Business, 2007
Lamb R A, Lai C J, Choppin P W. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci USA, 1981, 78:4170–4174 1:CAS:528:DyaL3MXlvF2hsro%3D, 10.1073/pnas.78.7.4170, 6945577
Sugrue R J, Belshe R B, Hay A J. Palmitoylation of the influenza A virus M2 protein. Virology, 1990, 179:51–56 1:CAS:528:DyaK3cXmt1Oktr0%3D, 10.1016/0042-6822(90)90272-S, 2219738
Holsinger L J, Lamb R A. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology, 1991, 183:32–43 1:CAS:528:DyaK3MXksVCmtL8%3D, 10.1016/0042-6822(91)90115-R, 2053285
Thomas J M, Stevens M P, Percy N, et al. Phosphorylation of the M2 protein of influenza A virus is not essential for virus viability. Virology, 1998, 252:54–64 1:CAS:528:DyaK1cXotFSitLY%3D, 10.1006/viro.1998.9384, 9875317
Horvath C M, Williams M A, Lamb R A. Eukaryotic coupled translation of tandem cistrons: identification of the influenza B virus BM2 polypeptide. EMBO J, 1990, 9:2639–2647 1:CAS:528:DyaK3cXlt1yhu7c%3D, 2114979
Odagiri T, Hong J, Ohara Y. The BM2 protein of influenza B virus is synthesized in the late phase of infection and incorporated into virions as a subviral component. J Gen Virol, 1999, 80:2573–2581 1:CAS:528:DyaK1MXmsVygsb4%3D, 10573149
Park E K, Castrucci M R, Portner A, et al. The M2 ectodomain is important for its incorporation into influenza A virions. J Virol, 1998, 72:2449–2455 1:CAS:528:DyaK1cXhtFegsbc%3D, 9499106
Paterson R G, Takeda M, Ohigashi Y, et al. Influenza B virus BM2 protein is an oligomeric integral membrane protein expressed at the cell surface. Virology, 2003, 306:7–17 1:CAS:528:DC%2BD3sXhs1Kntrg%3D, 10.1016/S0042-6822(02)00083-1, 12620792
Watanabe S, Imai M, Ohara Y, et al. Influenza B virus BM2 protein is transported through the trans-Golgi network as an integral membrane protein. J Virol, 2003, 77:10630–10637 1:CAS:528:DC%2BD3sXnsFymt7s%3D, 10.1128/JVI.77.19.10630-10637.2003, 12970447
Wang J, Pielak R M, McClintock M A, et al. Solution structure and functional analysis of the influenza B proton channel. Nat Struct Mol Biol, 2009, 16:1267–1271 1:CAS:528:DC%2BD1MXhtlOrurrO, 10.1038/nsmb.1707, 19898475
Sugrue R J, Hay A J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology, 1991, 180:617–624 1:CAS:528:DyaK3MXnvVeluw%3D%3D, 10.1016/0042-6822(91)90075-M, 1989386
Pinto L H, Holsinger L J, Lamb R A. Influenza virus M2 protein has ion channel activity. Cell, 1992, 69:517–528 1:CAS:528:DyaK38Xks1Cqtrg%3D, 10.1016/0092-8674(92)90452-I, 1374685
Henkel J R, Gibson G A, Poland P A, et al. Influenza M2 proton channel activity selectively inhibits trans-Golgi network release of apical membrane and secreted proteins in polarized Madin-Darby canine kidney cells. J Cell Biol, 2000, 148:495–504 1:CAS:528:DC%2BD3cXhtFSqtrc%3D, 10.1083/jcb.148.3.495, 10662775
Takeda M, Pekosz A, Shuck K, et al. Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture. J Virol, 2002, 76:1391–1399 1:CAS:528:DC%2BD38XnvFCqsg%3D%3D, 10.1128/JVI.76.3.1391-1399.2002, 11773413
Mould J A, Paterson R G, Takeda M, et al. Influenza B virus BM2 protein has ion channel activity that conducts protons across membranes. Dev Cell, 2003, 5:175–184 1:CAS:528:DC%2BD3sXmt1Smsb0%3D, 10.1016/S1534-5807(03)00190-4, 12852861
Pinto L H, Lamb R A. The M2 proton channels of influenza A and B viruses. J Biol Chem, 2006, 281:8997–9000 1:CAS:528:DC%2BD28XjtVSktrg%3D, 10.1074/jbc.R500020200, 16407184
McCown M F, Pekosz A. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J Virol, 2005, 79:3595–3605 1:CAS:528:DC%2BD2MXit1ehs7Y%3D, 10.1128/JVI.79.6.3595-3605.2005, 15731254
McCown M F, Pekosz A. Distinct domains of the influenza a virus M2 protein cytoplasmic tail mediate binding to the M1 protein and facilitate infectious virus production. J Virol, 2006, 80:8178–8189 1:CAS:528:DC%2BD28Xot1yrtLY%3D, 10.1128/JVI.00627-06, 16873274
Chen B J, Leser G P, Jackson D, et al. The influenza virus M2 protein cytoplasmic tail interacts with the M1 protein and influences virus assembly at the site of virus budding. J Virol, 2008, 82:10059–10070 1:CAS:528:DC%2BD1cXht1amtrvM, 10.1128/JVI.01184-08, 18701586
Hatta M, Goto H, Kawaoka Y. Influenza B virus requires BM2 pro tein for replication. J Virol, 2004, 78:5576–5583 1:CAS:528:DC%2BD2cXksFCgs7w%3D, 10.1128/JVI.78.11.5576-5583.2004, 15140954
Imai M, Watanabe S, Ninomiya A, et al. Influenza B virus BM2 protein is a crucial component for incorporation of viral ribonucleoprotein complex into virions during virus assembly. J Virol, 2004, 78:11007–11015 1:CAS:528:DC%2BD2cXotlKju70%3D, 10.1128/JVI.78.20.11007-11015.2004, 15452221
Zhou Z, Jiang X, Liu D, et al. Autophagy is involved in influenza A virus replication. Autophagy, 2009, 5:321–328 1:CAS:528:DC%2BD1MXnvVyku7Y%3D, 10.4161/auto.5.3.7406, 19066474
Rossman J S, Lamb R A. Autophagy, apoptosis, and the influenza virus M2 protein. Cell Host Microbe, 2009, 6:299–300 1:CAS:528:DC%2BD1MXhsVCjtr3J, 10.1016/j.chom.2009.09.009, 19837369
Skou J C, Esmann M. The Na,K-ATPase. J Bioenerg Biomembr, 1992, 24:249–261 1:CAS:528:DyaK38XltVKrsbg%3D, 1328174
Mobasheri A, Avila J, Cozar-Castellano I, et al. Na+,K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep, 2000, 20:51–91 1:CAS:528:DC%2BD3cXmtFymu7w%3D, 10.1023/A:1005580332144, 10965965
Emanuel J R, Garetz S, Stone L, et al. Differential expression of Na+,K+-ATPase alpha- and beta-subunit mRNAs in rat tissues and cell lines. Proc Natl Acad Sci USA, 1987, 84:9030–9034 1:CAS:528:DyaL1cXht1Gls78%3D, 10.1073/pnas.84.24.9030, 2827165
Miller R P, Farley R A. All three potential N-glycosylation sites of the dog kidney (Na++K+)-ATPase beta-subunit contain oligosaccharide. Biochim Biophys Acta, 1988, 954:50–57 1:CAS:528:DyaL1cXktVejsrc%3D, 2833926
Miller R P, Farley R A. Beta subunit of (Na++K+)-ATPase contains three disulfide bonds. Biochemistry, 1990, 29:1524–1532 1:CAS:528:DyaK3cXnt1yktQ%3D%3D, 10.1021/bi00458a025, 2159340
Noguchi S, Higashi K, Kawamura M. A possible role of the betasubunit of (Na,K)-ATPase in facilitating correct assembly of the alphasubunit into the membrane. J Biol Chem, 1990, 265:15991–15995 1:CAS:528:DyaK3cXls1yjsbY%3D, 2168428
Rajasekaran S A, Gopal J, Willis D, et al. Na,K-ATPase beta1-subunit increases the translation efficiency of the alpha1-subunit in MSV-MDCK cells. Mol Biol Cell, 2004, 15:3224–3232 1:CAS:528:DC%2BD2cXlslWnsL0%3D, 10.1091/mbc.E04-03-0222, 15133131
Shoshani L, Contreras R G, Roldan M L, et al. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between beta-subunits located in neighboring cells. Mol Biol Cell, 2005, 16:1071–1081 1:CAS:528:DC%2BD2MXit1Srsbs%3D, 10.1091/mbc.E04-03-0267, 15616198
Yoshimura S H, Iwasaka S, Schwarz W, et al. Fast degradation of the auxiliary subunit of Na+/K+-ATPase in the plasma membrane of HeLa cells. J Cell Sci, 2008, 121:2159–2168 1:CAS:528:DC%2BD1cXptF2jtbw%3D, 10.1242/jcs.022905, 18522992
Kitamura N, Ikekita M, Sato T, et al. Mouse Na+/K+-ATPase beta1-subunit has a K+-dependent cell adhesion activity for beta-GlcNActerminating glycans. Proc Natl Acad Sci USA, 2005, 102:2796–2801 1:CAS:528:DC%2BD2MXitVSksL0%3D, 10.1073/pnas.0409344102, 15705719
Rajasekaran S A, Palmer L G, Quan K, et al. Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell, 2001, 12:279–295 1:CAS:528:DC%2BD3MXhtlGjtL0%3D, 11179415
Pratscher B, Friedrich C, Goger W, et al. Characterization of NKIP: a novel, Na+/K+-ATPase interacting protein mediates neural differentiation and apoptosis. Exp Cell Res, 2008, 314:463–477 1:CAS:528:DC%2BD1cXpsFOruw%3D%3D, 10.1016/j.yexcr.2007.11.013, 18096156
Jha S, Dryer S E. The beta1 subunit of Na+/K+-ATPase interacts with BKCa channels and affects their steady-state expression on the cell surface. FEBS Lett, 2009, 583:3109–3114 1:CAS:528:DC%2BD1MXht1aisrnP, 10.1016/j.febslet.2009.08.039, 19729011
Kirley T L. Determination of three disulfide bonds and one free sulfhydryl in the beta subunit of (Na,K)-ATPase. J Biol Chem, 1989, 264:7185–7192 1:CAS:528:DyaL1MXktVeht7g%3D, 2540179
Gannage M, Dormann D, Albrecht R, et al. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe, 2009, 6:367–380 1:CAS:528:DC%2BD1MXhsVCjtrrN, 10.1016/j.chom.2009.09.005, 19837376
Rossman J S, Jing X, Leser G P, et al. Influenza virus m2 ion channel protein is necessary for filamentous virion formation. J Virol, 2010, 84:5078–5088 1:CAS:528:DC%2BC3cXmtVWgsL8%3D, 10.1128/JVI.00119-10, 20219914
Wilson P D, Devuyst O, Li X, et al. Apical plasma membrane mispolarization of NaK-ATPase in polycystic kidney disease epithelia is associated with aberrant expression of the beta2 isoform. Am J Pathol, 2000, 156:253–268 1:CAS:528:DC%2BD3cXns1yksg%3D%3D, 10623674
Ichinohe T, Pang I K, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol, 2010, 11:404–410 1:CAS:528:DC%2BC3cXksFaks7Y%3D, 10.1038/ni.1861, 20383149
Xie Z, Wang Y, Liu G, et al. Similarities and differences between the properties of native and recombinant Na+/K+-ATPases. Arch Biochem Biophys, 1996, 330:153–162 1:CAS:528:DyaK28Xjtlait7o%3D, 10.1006/abbi.1996.0237, 8651690
Noguchi S, Higashi K, Kawamura M. Assembly of the alpha-subunit of Torpedo californica Na+/K(+)-ATPase with its pre-existing beta-subunit in Xenopus oocytes. Biochim Biophys Acta, 1990, 1023:247–253 1:CAS:528:DyaK3cXitlWrtbw%3D, 10.1016/0005-2736(90)90420-S, 2158350
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mi, S., Li, Y., Yan, J. et al. Na+/K+-ATPase β1 subunit interacts with M2 proteins of influenza A and B viruses and affects the virus replication. Sci. China Life Sci. 53, 1098–1105 (2010). https://doi.org/10.1007/s11427-010-4048-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-010-4048-7