Abstract
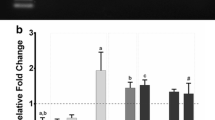
Compared with the understanding for the functional mechanism of the myostatin gene, little is known about the regulatory mechanism of the myostatin gene transcription and expression. To better understand the function of the myostatin gene promoter (MSTNpro) in the transcriptional regulation of the myostatin gene and to further investigate the transcriptional regulation mechanism of the myostatin gene, the promoter region of the myostatin gene in sheep has been cloned in our recent study (AY918121). In this study, the wild (W) type MSTNProW-EGFP vectors and E-box (E) (CANNTG) mutant (M) type MSTNProE(3+5+7)M-EGFP vectors were constructed and the transcriptional regulation activities were compared by detecting the fluorescent strength of EGFP (enhanced green fluorescent protein) in C2C12 myoblasts (or myotubes) and sheep fibroblasts transfected with the vectors. Results showed that the 0.3–1.2 kb sheep myostatin promoter could activate the transcription and expression of EGFP gene in C2C12 myoblasts to different extent and the 1.2 kb promoter was the strongest. However, fluorescence was not observed in the sheep fibroblasts transfected with the 1.2 kb sheep myostatin promoter. These results suggested that the specific nature of the myostatin gene expression in skeletal muscle was attributed to the specific nature of the myostatin promoter activity. The increasing growth density of C2C12 myoblasts inhibited the transcriptional regulation activity of the wild type sheep myostatin promoter by a mechanism of feedback. The transcriptional regulation activity of the 1.2 kb wild type sheep myostatin promoter increased significantly after C2C12 myoblasts were differentiated, while the activity of 1.2 kb E(3+5+7)-mutant type myostatin promoter had no obvious change. This result suggested that MyoD may be responsible for the difference of the myostatin gene transcription and expression between growing and differentiating conditions by binding to E-box of the myostatin promoter.
Similar content being viewed by others
References
McPherron A C, Lawler A M, Lee S J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature, 1997, 387: 83–90
Rios R, Fernandez-Nocelos S, Carneiro I, et al. Differential response to exogenous and endogenous myostatin in myoblasts suggests myostatin acts as an autocrine factor in vivo. Endocrinology, 2004, 145(6): 2795–2803
McPherron A C, Lee S J. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA, 1997, 94(23): 12457–12461
Kambadur R, Sharma M, Smith T P L, et al. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res, 1997, 7(9): 910–916
Grobet L, Martin L J, Poncelet D, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet, 1997, 17(1): 71–74
Grobet L, Poncelet D, Royo L J, et al. Molecular definition of an allele series of mutations disrupting the myostatin function and causing double-muscling in cattle. Mamm Genome, 1998, 9(3): 210–213
Bellinge R H S, Liberles D A, Laschi S P A, et al. Myostatin and its implications on animal breeding: A review. Anim Genet, 2005, 36(1): 1–6
Schuelke M, Wagner K R, Stolz L E, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med, 2004, 350(26): 2682–2688
Lee S J, McPherron A C. Regulation of Myostatin activity and muscle growth. Proc Natl Acad Sci USA, 2001, 98(16): 9306–9311
Langley B, Thomas M, Bishop A, et al. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem, 2002, 277(51): 49831–49840
Zhu X, Topouzis S, Liang L F, et al. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine, 2004, 26(6): 262–272
Forbes D, Jackman M, Bishop A, et al. Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol, 2006, 206(1): 264–272
Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem, 2000, 275(51): 40235–40243
Tellgren A, Berglund A C, Savolainen P, et al. Myostatin rapid sequence evolution in ruminants predates domestication. Mol Phylogenet Evol, 2004, 33: 782–790
Gu Z, Zhang Y, Shi P, et al. Comparison of avian myostatin genes. Anim Genet, 2004, 35(6): 470–472
Ostbye T K, Galloway T F, Nielsen C, et al. The two myostatin genes of Atlantic salmon (Salmo salar) are expressed in a variety of tissues. Eur J Biochem, 2001, 268(20): 5249–5257
Xu C, Wu G, Zohar Y, et al. Analysis of myostatin gene structure, expression and function in zebrafish. J Exp Biol, 2003, 206: 4067–4079
Amali A A, Lin C J F, Chen Y H, et al. Up-regulation of muscle-specific transcription factors during embryonic somitogenesis of zebrafish (danio rerio) by knock-down of myostatin-1. Dev Dyn, 2004, 229(4): 847–856
Martin C I, Johnston I A. The role of myostatin and the calcineurin-signalling pathway in regulating muscle mass in response to exercise training in the rainbow trout Oncorhynchus mykiss Walbaum. J Exp Biol, 2005, 208: 2083–2090
Kerr T, Roalson E H, Rodgers B D. Phylogenetic analysis of the myostatin gene sub-family and the differential expression of a novel member in zebrafish. Evol Dev, 2005, 7(5): 390–400
Oldham J M, Martyn J A, Sharma M, et al. Molecular expression of myostatin and MyoD is greater in double-muscled than normal-muscled cattle fetuses. Am J Physiol Regul Integr Comp Physiol, 2001, 280(5): R1488–R1493
Ma K, Mallidis C, Artaza J, et al. Characterization of 5′-regulatory region of human myostatin gene: Regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab, 2001, 281(6): E1128–E1136
Allen D L, Unterman T G. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol, 2007, 292: C188–C199
Spiller M P, Kambadur R, Jeanplong F, et al. The myostatin gene is a downstream target gene of basic helix-loop-helix transcription factor MyoD. Mol Cell Biol, 2002, 22(20): 7066–7082
Crisa A, Marchitelli C, Savarese M C, et al. Sequence analysis of myostatin promoter in cattle. Cytogenet Genome Res, 2003, 102(1–4): 48–52
Salerno M S, Thomas M, Forbes D, et al. Molecular analysis of fiber type-specific expression of murine myostatin promoter. Am J Physiol Cell Physiol, 2004, 287(4): C1031–C1040
Du R, Chen Y F, An X R, et al. Cloning and sequence analysis of myostatin promoter in sheep. DNA Seq, 2005, 16(6): 412–417
Yu Z Q, Li Y, Meng Q Y, et al. Comparative analysis of the pig BAC sequence involved in the regulation of myostatin gene. Sci China Ser C-Life Sci, 2005, 48(2): 168–180
Radaelli G, Rowlerson A, Mascarello F, et al. Myostatin precursor is present in several tissues in teleost fish: A comparative immunolocalization study. Cell Tissue Res, 2003, 311(2): 239–250
Roberts S B, Goetz F W. Differential skeletal muscle expression of myostatin across teleost species, and the isolation of multiple myostatin isoforms. FEBS Lett, 2001, 491(3): 212–216
Ji S, Losinski R L, Cornelius S G, et al. Myostatin expression in porcine tissues: tissue specificity and developmental and postnatal regulation. Am J Physiol Regul Integr Comp Physiol, 1998, 275(4): R1265–1273
Sharma M, Kambadur R, Matthews K G, et al. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol, 1999, 180(1): 1–9
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Hi-Tech R&D Program (“863” Program) of China (Grant No. 2002AA206311)
Rights and permissions
About this article
Cite this article
Du, R., An, X., Chen, Y. et al. Functional analysis of the Myostatin gene promoter in sheep. SCI CHINA SER C 50, 648–654 (2007). https://doi.org/10.1007/s11427-007-0085-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11427-007-0085-2