Abstract
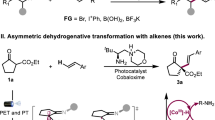
Catalytic amination of alkenes is one of the most attractive reactions for the construction of complex heterocycles with nitrogen centers. Herein, we present that synergistic photoredox and cobaloxime catalysis allows for highly efficient and mild dehydrogenative reactions between various NH nucleophiles and di-, tri-, and tetrasubstituted alkenes in the absence of external oxidants, thus enabling access to an array of N-heterocycles. Notably, both Z- and E-alkene-containing N-heterocycles are accessible. Mechanistic studies indicated that the Z-cinnamyl derivatives could be generated by photocatalytic E to Z alkene isomerization through an energy transfer process. Moreover, we find that sluggish energy transfer could inhibit the E to Z alkene isomerization process, thus offering the cinnamyl derivatives with E-selectivity. Our results highlight the benefits of the reactions using dual photoredox and cobaloxime catalysis to lead to diverse N-heterocycles.

Similar content being viewed by others
Change history
20 September 2022
An Erratum to this paper has been published: https://doi.org/10.1007/s11426-022-1393-1
References
Nay B, Riache N, Evanno L. Nat Prod Rep, 2009, 26: 1044–1062
Lovering F, Bikker J, Humblet C. J Med Chem, 2009, 52: 6752–6756
Vitaku E, Smith DT, Njardarson JT. J Med Chem, 2014, 57: 10257–10274
For some reviews, see: (a) Kotov V, Scarborough CC, Stahl SS. Inorg Chem, 2007, 46: 1910–1923
McDonald RI, Liu G, Stahl SS. Chem Rev, 2011, 111: 2981–3019
Kočovský P, Bäckvall JE. Chem Eur J, 2015, 21: 36–56
For selected examples, see: (a) Larock RC, Hightower TR, Hasvold LA, Peterson KP. J Org Chem, 1996, 61: 3584–3585
Fix SR, Brice JL, Stahl SS. Angew Chem Int Ed, 2002, 41: 164–166
McDonald RI, Stahl SS. Angew Chem Int Ed, 2010, 49: 5529–5532
McDonald RI, White PB, Weinstein AB, Tam CP, Stahl SS. Org Lett, 2011, 13: 2830–2833
Weinstein AB, Stahl SS. Angew Chem Int Ed, 2012, 51: 11505–11509
Yang G, Shen C, Zhang W. Angew Chem Int Ed, 2012, 51: 9141–9145
Weinstein AB, Schuman DP, Tan ZX, Stahl SS. Angew Chem Int Ed, 2013, 52: 11867–11870
Kou X, Shao Q, Ye C, Yang G, Zhang W. J Am Chem Soc, 2018, 140: 7587–7597
Xiong P, Xu HH, Xu HC. J Am Chem Soc, 2017, 139: 2956–2959
Huang C, Li ZY, Song J, Xu HC. Angew Chem Int Ed, 2021, 60: 11237–11241
Cai CY, Wu ZJ, Liu JY, Chen M, Song J, Xu HC. Nat Commun, 2021, 12: 3745
Yi X, Hu X. Angew Chem Int Ed, 2019, 58: 4700–4704
Xiong P, Xu F, Qian XY, Yohannes Y, Song J, Lu X, Xu HC. Chem Eur J, 2016, 22: 4379–4383
Yang D, Chen J, Huang Y, Pan H, Shi J, Zhang Y, Wang F, Li Z. ACS Catal, 2021, 11: 9860–9868
Reed NL, Lutovsky GA, Yoon TP. J Am Chem Soc, 2021, 143: 6065–6070
For some reviews, see: (a) Prier CK, Rankic DA, MacMillan DWC. Chem Rev, 2013, 113: 5322–5363
Ravelli D, Protti S, Fagnoni M. Chem Rev, 2016, 116: 9850–9913
Romero NA, Nicewicz DA. Chem Rev, 2016, 116: 10075–10166
Zhou QQ, Zou YQ, Lu LQ, Xiao WJ. Angew Chem Int Ed, 2019, 58: 1586–1604
Miller DC, Choi GJ, Orbe HS, Knowles RR. J Am Chem Soc, 2015, 137: 13492–13495
Choi GJ, Knowles RR. J Am Chem Soc, 2015, 137: 9226–9229
For Ag-catalyzed amidyl radical cyclization, see: Li Z, Song L, Li C. J Am Chem Soc, 2013, 135: 4640–4643
Jia J, Ho YA, Bülow RF, Rueping M. Chem Eur J, 2018, 24: 14054–14058
Zheng S, Gutiérrez-Bonet Á, Molander GA. Chem, 2019, 5: 339–352
Zhang C, Wang Y, Song Y, Gao H, Sun Y, Sun X, Yang Y, He M, Yang Z, Zhan L, Yu ZX, Rao Y. CCS Chem, 2019, 1: 352–364
Zhu Q, Graff DE, Knowles RR. J Am Chem Soc, 2018, 140: 741–747
Giedyk M, Goliszewska K, Gryko D. Chem Soc Rev, 2015, 44: 3391–3404
Weiss ME, Kreis LM, Lauber A, Carreira EM. Angew Chem Int Ed, 2011, 50: 11125–11128
Kreis LM, Krautwald S, Pfeiffer N, Martin RE, Carreira EM. Org Lett, 2013, 15: 1634–1637
Liu WQ, Lei T, Zhou S, Yang XL, Li J, Chen B, Sivaguru J, Tung CH, Wu LZ. J Am Chem Soc, 2019, 141: 13941–13947
Zhang G, Zhang L, Yi H, Luo Y, Qi X, Tung CH, Wu LZ, Lei A. Chem Commun, 2016, 52: 10407–10410
Cao H, Jiang H, Feng H, Kwan JMC, Liu X, Wu J. J Am Chem Soc, 2018, 140: 16360–16367
Sun X, Chen J, Ritter T. Nat Chem, 2018, 10: 1229–1233
Tu JL, Liu JL, Tang W, Su M, Liu F. Org Lett, 2020, 22: 1222–1226
Tu JL, Tang W, Xu W, Liu F. J Org Chem, 2021, 86: 2929–2940
For reviews, see: (a) Molloy JJ, Morack T, Gilmour R. Angew Chem Int Ed, 2019, 58: 13654–13664
Zhang H, Yu S. Chin J Org Chem, 2019, 39: 95–108
Neveselý T, Wienhold M, Molloy JJ, Gilmour R. Chem Rev, 2022, 122: 2650–2694
Cheng X, Li T, Liu Y, Lu Z. ACS Catal, 2021, 11: 11059–11065
Xu J, Li Z, Xu Y, Shu X, Huo H. ACS Catal, 2021, 11: 13567–13574
Singh K, Staig SJ, Weaver JD. J Am Chem Soc, 2014, 136: 5275–5278
Metternich JB, Gilmour R. J Am Chem Soc, 2015, 137: 11254–11257
Brégent T, Bouillon JP, Poisson T. Org Lett, 2020, 22: 7688–7693
Shen X, Huang C, Yuan XA, Yu S. Angew Chem Int Ed, 2021, 60: 9672–9679
Gellert E. J Nat Prod, 1982, 45: 50–73
Gao W, Lam W, Zhong S, Kaczmarek C, Baker DC, Cheng YC. Cancer Res, 2004, 64: 678–688
Wen T, Wang Z, Meng X, Wu M, Li Y, Wu X, Zhao L, Wang P, Yin Z, Li-Ling J, Wang Q. ACS Med Chem Lett, 2014, 5: 1027–1031
Han G, Chen L, Wang Q, Wu M, Liu Y, Wang Q. J Agric Food Chem, 2018, 66: 780–788
Newcomb M, Esker JL. Tetrahedron Lett, 1991, 32: 1035–1038
Orito K, Miyazawa M, Nakamura T, Horibata A, Ushito H, Nagasaki H, Yuguchi M, Yamashita S, Yamazaki T, Tokuda M. J Org Chem, 2006, 71: 5951–5958
Bertrand MB, Leathen ML, Wolfe JP. Org Lett, 2007, 9: 457–460
Dempsey JL, Brunschwig BS, Winkler JR, Gray HB. Acc Chem Res, 2009, 42: 1995–2004
Ruccolo S, Qin Y, Schnedermann C, Nocera DG. J Am Chem Soc, 2018, 140: 14926–14937
A hydrogen atom abstraction process involving Co(II) complex, see: Zhao H, McMillan AJ, Constantin T, Mykura RC, Juliá F, Leonori D. J Am Chem Soc, 2021, 143: 14806–14813
Acknowledgements
This work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA350001) and the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Conflict of interest
The authors declare no conflict of interest.
The online version of the original article can be found at https://doi.org/10.1007/s11426-022-1393-1
Supporting Information For
Rights and permissions
About this article
Cite this article
Tu, JL., Tang, W., He, SH. et al. Acceptorless dehydrogenative amination of alkenes for the synthesis of N-heterocycles. Sci. China Chem. 65, 1330–1337 (2022). https://doi.org/10.1007/s11426-022-1241-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1241-x