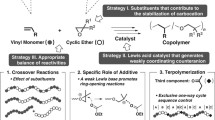
Abstract
The properties of poly(vinyl ether)s (PVEs) are highly dependent on their tacticity, and the appealing thermoplastics features of isotactic PVEs have drawn considerable efforts to develop stereoselective cationic polymerization methods to access this class of polymers. However, reported methods that could achieve a high degree of tacticity control are limited to process employing metal-based Lewis acids, and with various limitations on catalyst loading, monomer scope, etc. Here, we introduce a metal-free stereoselective cationic polymerization of vinyl ethers by employing a class of chiral confined Brønsted acids, imidodiphosphorimidates (IDPis), as the catalyst. This organocatalytic approach features its metal free conditions, high efficiency, high stereoselectivity, single catalyst system, operation simplicity, etc.

Similar content being viewed by others
References
Worch JC, Prydderch H, Jimaja S, Bexis P, Becker ML, Dove AP. Nat Rev Chem, 2019, 3: 514–535
Busico V. Giulio Natta and the Development of Stereoselective Propene Polymerization. In: Kaminsky W, Eds. Polyolefins: 50 years after Ziegler and Natta I. Advances in Polymer Science, Vol 257. Berlin, Heidelberg: Springer, 2013. 37–58
de Derosa C, Auriemma F. Prog Polym Sci, 2006, 31: 145–237
Fishbein L, Crowe BF. Makromol Chem, 1961, 48: 221–228
Teator AJ, Leibfarth FA. Science, 2019, 363: 1439–1443
Teator AJ, Varner TP, Knutson PC, Sorensen CC, Leibfarth FA. ACS Macro Lett, 2020, 9: 1638–1654
Aoshima S, Kanaoka S. Chem Rev, 2009, 109: 5245–5287
Sawamoto M. Prog Polym Sci, 1991, 16: 111–172
Coates GW. Chem Rev, 2000, 100: 1223–1252
Chen EYX. Chem Rev, 2009, 109: 5157–5214
Wang XY, Sun XL, Wang F, Tang Y. ACS Catal, 2017, 7: 4692–4696
Aoshima S, Ito Y, Kobayashi E. Polym J, 1993, 25: 1161–1168
Kamigaito M, Maeda Y, Sawamoto M, Higashimura T. Macromolecules, 1993, 26: 1643–1649
Ouchi M, Kamigaito M, Sawamoto M. Macromolecules, 1999, 32: 6407–6411
Ouchi M, Kamigaito M, Sawamoto M. J Polym Sci Polym Chem, 2001, 39: 1060–1066
Ouchi M, Sueoka M, Kamigaito M, Sawamoto M. J Polym Sci Polym Chem, 2001, 39: 1067–1074
Kawaguchi T, Sanda F, Masuda T. J Polym Sci Polym Chem, 2002, 40: 3938–3943
Sudhakar P, Vijayakrishna K. ChemCatChem, 2010, 2: 649–652
Kanazawa A, Kanaoka S, Aoshima S. J Polym Sci Polym Chem, 2010, 48: 3702–3708
Teator AJ, Varner TP, Jacky PE, Sheyko KA, Leibfarth FA. ACS Macro Lett, 2019, 8: 1559–1563
Watanabe H, Yamamoto T, Kanazawa A, Aoshima S. Polym Chem, 2020, 11: 3398–3403
Varner TP, Teator AJ, Reddi Y, Jacky PE, Cramer CJ, Leibfarth FA. J Am Chem Soc, 2020, 142: 17175–17186
Uchiyama M, Satoh K, Kamigaito M. Giant, 2021, 5: 100047
Shanmugam S, Boyer C. Science, 2016, 352: 1053–1054
Albertsson AC, Varma IK. Biomacromolecules, 2003, 4: 1466–1486
Sugihara S, Tanabe Y, Kitagawa M, Ikeda I. J Polym Sci Polym Chem, 2008, 46: 1913–1918
Uchiyama M, Satoh K, Kamigaito M. Angew Chem Int Ed, 2015, 54: 1924–1928
Sugihara S, Konegawa N, Maeda Y. Macromolecules, 2015, 48: 5120–5131
Song J, Xu J, Tang D. J Polym Sci Part A-Polym Chem, 2016, 54: 1373–1377
Kottisch V, Jermaks J, Mak JY, Woltornist RA, Lambert TH, Fors BP. Angew Chem Int Ed, 2021, 60: 4535–4539
Liao S, Čorić I, Wang Q, List B. J Am Chem Soc, 2012, 134: 10765–10768
Schreyer L, Properzi R, List B. Angew Chem Int Ed, 2019, 58: 12761–12777
Čorić I, List B. Nature, 2012, 483: 315–319
Kaib PSJ, Schreyer L, Lee S, Properzi R, List B. Angew Chem Int Ed, 2016, 55: 13200–13203
Xie Y, Cheng GJ, Lee S, Kaib PSJ, Thiel W, List B. J Am Chem Soc, 2016, 138: 14538–14541
Liu L, Kim H, Xie Y, Farès C, Kaib PSJ, Goddard R, List B. J Am Chem Soc, 2017, 139: 13656–13659
Lee S, Kaib PSJ, List B. J Am Chem Soc, 2017, 139: 2156–2159
Schreyer L, Kaib PSJ, Wakchaure VN, Obradors C, Properzi R, Lee S, List B. Science, 2018, 362: 216–219
Tsuji N, Kennemur JL, Buyck T, Lee S, Prévost S, Kaib PSJ, Bykov D, Farès C, List B. Science, 2018, 359: 1501–1505
Ouyang J, Kennemur JL, De CK, Farès C, List B. J Am Chem Soc, 2019, 141: 3414–3418
Ghosh S, Das S, De CK, Yepes D, Neese F, Bistoni G, Leutzsch M, List B. Angew Chem Int Ed, 2020, 59: 12347–12351
Zhang X, Jiang Y, Ma Q, Hu S, Wang Q, Liao S. Eur Polym J, 2020, 123: 109449
Mitschke B, Turberg M, List B. Chem, 2020, 6: 2515–2532
Schwengers SA, De CK, Grossmann O, Grimm JAA, Sadlowski NR, Gerosa GG, List B. J Am Chem Soc, 2021, 143: 14835–14844
Mahlau M, List B. Angew Chem Int Ed, 2013, 52: 518–533
Brak K, Jacobsen EN. Angew Chem Int Ed, 2013, 52: 534–561
Phipps RJ, Hamilton GL, Toste FD. Nat Chem, 2012, 4: 603–614
Liao S, List B. Angew Chem Int Ed, 2010, 49: 628–631
Merten C, Pollok CH, Liao S, List B. Angew Chem Int Ed, 2015, 54: 8841–8845
A patent (CN 202110033389.X) has been filed on this stereoselective polymerization method employing chiral confined Brønsted acids as the catalyst on 2021-01-12. This manuscript was first published as a preprint on ChemRxiv (Cambridge: Cambridge Open Engage, 2021), doi: https://doi.org/10.33774/chemrxiv-2021-qlpbp on 2021-10-04
For a similar work concurrently published on 2021-10-01, see: Knutson PC, Teator AJ, Varner TP, Kozuszek CT, Jacky PE, Leibfarth FA. J Am Chem Soc, 2021, 143: 16388–16393
Acknowledgements
This work was supported by the Recruitment Program of Global Experts, Beijing National Laboratory for Molecular Sciences (BNLMS201913), and Fuzhou University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2021_1143_MOESM1_ESM.pdf
Organocatalytic Stereoselective Cationic Polymerization of Vinyl Ethers by Employing a Confined Brønsted Acid as the Catalyst
Rights and permissions
About this article
Cite this article
Yang, Z., Zhang, X., Jiang, Y. et al. Organocatalytic stereoselective cationic polymerization of vinyl ethers by employing a confined brønsted acid as the catalyst. Sci. China Chem. 65, 304–308 (2022). https://doi.org/10.1007/s11426-021-1143-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1143-x