Abstract
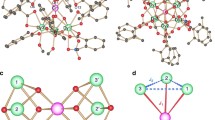
Two tetraazacyclophane dications (12+ and 22+) with different remote substituents have been synthesized, isolated and characterized. Their electronic structures and physical property were studied by various spectroscopic techniques, single crystal X-ray diffraction, super conducting quantum interference device (SQUID) measurements and theoretical calculations. The dications have triplet ground states with ferromagnetic interaction exceeding the thermal energy at room temperature. The solid-state structures of these species were tunable by substituent effect, with 12+ as a monomer and 22+ as a dimer.
Similar content being viewed by others
References
Salem L, Rowland C. Angew Chem Int Ed, 1972, 11: 92–111
Borden WT. Diradicals. New York: Wiley, 1982
Rajca A. Chem Rev, 1994, 94: 871–893
Schmittel M, Burghart A. Angew Chem Int Ed, 1997, 36: 2550–2589
Power PP. Chem Rev, 2003, 103: 789–810
Nishinaga T, Komatsu K. Org Biomol Chem, 2005, 3: 561–569
Hicks RG. Org Biomol Chem, 2007, 5: 1321–1338
Breher F. Coordin Chem Rev, 2007, 251: 1007–1043
Hicks RG. Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. Chichester, U.K.: Wiley, 2010
Martin D, Soleilhavoup M, Bertrand G. Chem Sci, 2011, 2: 389–399
Hankache J, Wenger OS. Chem Rev, 2011, 111: 5138–5178
Mas-Torrent M, Crivillers N, Rovira C, Veciana J. Chem Rev, 2012, 112: 2506–2527
Heckmann A, Lambert C. Angew Chem Int Ed, 2012, 51: 326–392
Abe M, Ye J, Mishima M. Chem Soc Rev, 2012, 41: 3808–3820
Sun Z, Ye Q, Chi C, Wu J. Chem Soc Rev, 2012, 41: 7857–7889
Casado J, Ponce Ortiz R, López Navarrete JT. Chem Soc Rev, 2012, 41: 5672–5686
Chivers T, Konu J. In: Chivers T, Ed. Comprehensive Inorganic Chemistry II: From Elements to Applications. Volume 1: Main-Group Elements, Including Noble Gases. Amsterdam: Elsevier, 2013. 349–373
Abe M. Chem Rev, 2013, 113: 7011–7088
Rassat A, Sieveking HU. Angew Chem Int Ed, 1972, 11: 303–304
Veciana J, Rovira C, Crespo MI, Armet O, Domingo VM, Palacio F. J Am Chem Soc, 1991, 113: 2552–2561
Inoue K, Iwamura H. Angew Chem Int Ed, 1995, 34: 927–928
Shultz DA, Fico RM, Lee H, Kampf JW, Kirschbaum K, Pinkerton AA, Boyle PD. J Am Chem Soc, 2003, 125: 15426–15432
Hiraoka S, Okamoto T, Kozaki M, Shiomi D, Sato K, Takui T, Okada K. J Am Chem Soc, 2004, 126: 58–59
Fukuzaki E, Nishide H. J Am Chem Soc, 2006, 128: 996–1001
Rajca A, Takahashi M, Pink M, Spagnol G, Rajca S. J Am Chem Soc, 2007, 129: 10159–10170
Rajca A, Shiraishi K, Rajca S. Chem Commun, 2009, 294: 4372
Boratyński PJ, Pink M, Rajca S, Rajca A. Angew Chim Int Ed, 2010, 49: 5459–5462
Suzuki S, Furui T, Kuratsu M, Kozaki M, Shiomi D, Sato K, Takui T, Okada K. J Am Chem Soc, 2010, 132: 15908–15910
Gallagher NM, Bauer JJ, Pink M, Rajca S, Rajca A. J Am Chem Soc, 2016, 138: 9377–9380
Bredas JL, Street GB. Acc Chem Res, 1985, 18: 309–315
Spangler CW, He M. J Chem Soc Perkin Trans 1, 1995, 715–720
de Melo CP, Silbey R. J Chem Phys, 1988, 88: 2567–2571
Lai CM, Meng HF. Phys Rev B, 1996, 54: 16365–16368
Bulovic V, Gu G, Burrows PE, Forrest SR, Thompson ME. Nature, 1996, 380: 29–29
Tamoto N, Adachi C, Nagai K. Chem Mater, 1997, 9: 1077–1085
Bellmann E, Shaheen SE, Thayumanavan S, Barlow S, Grubbs RH, Marder SR, Kippelen B, Peyghambarian N. Chem Mater, 1998, 10: 1668–1676
Borsenberger PM, Weiss DS. Organic Photoreceptors for Xerography. New York: Marcel Dekker, 1998
Harris MC, Buchwald SL. J Org Chem, 2000, 65: 5327–5333
Mitschke U, Bäuerle P. J Mater Chem, 2000, 10: 1471–1507
Nelsen SF, Ismagilov RF, Powell DR. J Am Chem Soc, 1997, 119: 10213–10222
Nelsen SF, Li G, Schultz KP, Tran HQ, Guzei IA, Evans DH. J Am Chem Soc, 2008, 130: 11620–11622
Jalilov AS, Li G, Nelsen SF, Guzei IA, Wu Q. J Am Chem Soc, 2010, 132: 6176–6182
Jalilov AS, Nelsen SF, Guzei IA, Wu Q. Angew Chem Int Ed, 2011, 50: 6860–6863
Yokoyama Y, Sakamaki D, Ito A, Tanaka K, Shiro M. Angew Chem Int Ed, 2012, 51: 9403–9406
Zalibera M, Jalilov AS, Stoll S, Guzei IA, Gescheidt G, Nelsen SF. J Phys Chem A, 2013, 117: 1439–1448
Kamada K, Fuku-en S, Minamide S, Ohta K, Kishi R, Nakano M, Matsuzaki H, Okamoto H, Higashikawa H, Inoue K, Kojima S, Yamamoto Y. J Am Chem Soc, 2013, 135: 232–241
Zheng X, Wang X, Qiu Y, Li Y, Zhou C, Sui Y, Li Y, Ma J, Wang X. J Am Chem Soc, 2013, 135: 14912–14915
Su Y, Wang X, Zheng X, Zhang Z, Song Y, Sui Y, Li Y, Wang X. Angew Chem Int Ed, 2014, 53: 2857–2861
Su Y, Wang X, Li Y, Song Y, Sui Y, Wang X. Angew Chem Int Ed, 2015, 54: 1634–1637
Li T, Tan G, Shao D, Li J, Zhang Z, Song Y, Sui Y, Chen S, Fang Y, Wang X. J Am Chem Soc, 2016, 138: 10092–10095
Su Y, Wang X, Wang L, Zhang Z, Wang X, Song Y, Power PP. Chem Sci, 2016, 7: 6514–6518
Burrezo PM, Lin NT, Nakabayashi K, Ohkoshi SI, Calzado EM, Boj PG, Díaz García MA, Franco C, Rovira C, Veciana J, Moos M, Lambert C, López Navarrete JT, Tsuji H, Nakamura E, Casado J. Angew Chem Int Ed, 2017, 56: 2898–2902
Ito A, Urabe M, Tanaka K. Angew Chem Int Ed, 2003, 42: 921–924
Ito A, Urabe M, Tanaka K. Angew Chem Int Ed, 2009, 48: 5785–5785
Wienk MM, Janssen RAJ. J Am Chem Soc, 1996, 118: 10626–10628
Fukuzaki E, Nishide H. J Am Chem Soc, 2006, 128: 996–1001
Hauck SI, Lakshmi KV, Hartwig JF. Org Lett, 1999, 1: 2057–2060
Ito A, Ono Y, Tanaka K. Angew Chem Int Ed, 2000, 39: 1072−1075
Kulszewicz-Bajer I, Maurel V, Gambarelli S, Wielgus I, Djurado D. Phys Chem Chem Phys, 2009, 11: 1362–1368
Tsue H, Ishibashi K, Tamura R. Top Heterocycl Chem, 2008, 17: 73–96
Wang MX. Chem Commun, 2008, 27: 4541
Wang MX. Acc Chem Res, 2012, 45: 182–195
Bujak P, Kulszewicz-Bajer I, Zagorska M, Maurel V, Wielgus I, Pron A. Chem Soc Rev, 2013, 42: 8895–8999
Ito A. J Mater Chem C, 2016, 4: 4614–4625
Spin contamination errors were corrected by approximate spin-projection method. S2 before spin projection were 2.0267 and 2.0231 for the triplet states; 1.0045 and 1.0016 for the open-shell singlet states of 1 2+ and 2 2+, respectively. Kitagawa Y, Saito T, Ito M, Shoji M, Koizumi K, Yamanaka S, Kawakami T, Okumura M, Yamaguchi K. Chem Phys Lett, 2007, 442: 445–450
Krossing I. Chem Eur J, 2001, 7: 490–502
X-ray data for 1 2+ and 2 2+ are listed in Table S1 in the Supporting Information online. CCDC-1534033 and 1543240 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
All calculations were performed using the Gaussian 09 program suite. Frisch MJ, et al. Gaussian 09. Revision B.01. Wallingford, CT: Gaussian, Inc., 2010. See Supporting Information for coordinates and full citation
Bondi A. J Phys Chem, 1964, 68: 441–451
Haldane FDM. Phys Lett A, 1983, 93: 464–468
Haldane FDM. Phys Rev Lett, 1983, 50: 1153–1156
Zheludev A, Honda Z, Chen Y, Broholm CL, Katsumata K, Shapiro SM. Phys Rev Lett, 2002, 88: 077206
Stone MB, Zaliznyak IA, Hong T, Broholm CL, Reich DH. Nature, 2006, 440: 187–190
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21525102, 21690062), and the Natural Science Foundation of Jiangsu Province (BK20140014). We are grateful to the High Performance Computing Center of Nanjing University for doing the numerical calculations in this paper on its IBM Blade cluster system.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11426_2017_9101_MOESM1_ESM.doc
Air-Stable Diradical Dications with Ferromagnetic Interaction Exceeding the Thermal Energy at Room Temperature: From a Monomer to a Dimer
Rights and permissions
About this article
Cite this article
Wang, W., Wang, L., Chen, S. et al. Air-stable diradical dications with ferromagnetic interaction exceeding the thermal energy at room temperature: from a monomer to a dimer. Sci. China Chem. 61, 300–305 (2018). https://doi.org/10.1007/s11426-017-9101-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-017-9101-5