Abstract
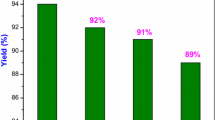
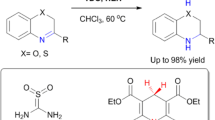
N-methyl-tetrahydroquinolines (MTHQs) are a kind of very useful chemicals, which can be obtained from N-methylation of amines. However, the methylation of quinolines which is a kind of highly unsaturated nitrogen-containing heterocyclic aromatic compounds has not been reported. In this work, we report the first work for the synthesis of MTHQs by methylation of quinolines using CO2 and H2. It was found that Ru(acac)3-triphos [triphos: 1,1,1-tris(diphenylphosphinomethyl)ethanl] complex was very active and selective for the N-methylation reaction of quinolines, and the yield of the desired product could reach 99%.
Similar content being viewed by others
References
Páez DE, Andriollo A, Morfes G. Catal Today, 2015, 247: 139–146
Fang M, Sánchez-Delgado RA. J Catal, 2014, 311: 357–368
Deepa G, Sankaranarayanan TM, Shanthi K, Viswanathan B. Catal Today, 2012, 198: 252–262
Schmitter JM, Ignatiadis I, Arpino PJ. Geochim Cosmochim Acta, 1983, 47: 1975–1984
Sridharan V, Suryavanshi PA, Menéndez JC. Chem Rev, 2011, 111: 7157–7259
Jacquemond-Collet I, Hannedouche S, Fabre N, Fouraste I, Moulis C. Phytochemistry, 1999, 51: 1167–1169
O’Byrne A, Evans P. Tetrahedron, 2008, 64: 8067–8072
Theeraladanon C, Arisawa M, Nakagawa M, Nishida A. Tetrahedron-Asymmetry, 2005, 16: 827–831
Wang Y, Liu Y, Zhang D, Wei H, Shi M, Wang F. Angew Chem Int Ed, 2016, 55: 3776–3780
Pappoppula M, Cardoso FSP, Garrett BO, Aponick A. Angew Chem Int Ed, 2015, 54: 15202–15206
Muñoz GD, Dudley GB. Org Prep Proced Int, 2015, 47: 179–206
Ye KY, He H, Liu WB, Dai LX, Helmchen G, You SL. J Am Chem Soc, 2011, 133: 19006–19014
Bentley SA, Davies SG, Lee JA, Roberts PM, Thomson JE. Org Lett, 2011, 13: 2544–2547
Kothandaraman P, Foo SJ, Chan PWH. J Org Chem, 2009, 74: 5947–5952
Lei P, Xu Y, Du J, Yang XL, Yuan HZ, Xu GF, Ling Y. Bioorg Med Chem Lett, 2016, 26: 2544–2546
Yang PY, Zhou YG. Tetrahedron-Asymmetry, 2004, 15: 1145–1149
Taylor LL, Goldberg FW, Hii KKM. Org Biomol Chem, 2012, 10: 4424–4432
Chen F, Surkus AE, He L, Pohl MM, Radnik J, Topf C, Junge K, Beller M. J Am Chem Soc, 2015, 137: 11718–11724
Zhang Z, Du H. Org Lett, 2015, 17: 6266–6269
Karakulina A, Gopakumar A, Akçok, Roulier BL, LaGrange T, Katsyuba SA, Das S, Dyson PJ. Angew Chem Int Ed, 2016, 55: 292–296
Ren D, He L, Yu L, Ding RS, Liu YM, Cao Y, He HY, Fan KN. J Am Chem Soc, 2012, 134: 17592–17598
Wang WB, Lu SM, Yang PY, Han XW, Zhou YG. J Am Chem Soc, 2003, 125: 10536–10537
Tu XF, Gong LZ. Angew Chem Int Ed, 2012, 51: 11346–11349
Tummatorn J, Muñoz GD, Dudley GB. Tetrahedron Lett, 2013, 54: 1312–1314
Sorribes I, Junge K, Beller M. Chem Eur J, 2014, 20: 7878–7883
Dang TT, Ramalingam B, Seayad AM. ACS Catal, 2015, 5: 4082–4088
Abarca B, Adam R, Ballesteros R. Org Biomol Chem, 2012, 10: 1826–1833
Babu TH, Shanthi G, Perumal PT. Tetrahedron Lett, 2009, 50: 2881–2884
Qian Q, Zhang J, Cui M, Han B. Nat Commun, 2016, 7: 11481
He Z, Qian Q, Ma J, Meng Q, Zhou H, Song J, Liu Z, Han B. Angew Chem, 2016, 128: 747–751
Wang MY, Ma R, He LN. Sci China Chem, 2016, 59: 507–516
Börjesson M, Moragas T, Martin R. J Am Chem Soc, 2016, 138: 7504–7507
Zhang S, Li X, He L. Acta Chim Sinica, 2016, 74: 7–23
He M, Sun Y, Han B. Angew Chem Int Ed, 2013, 52: 9620–9633
Beydoun K, Ghattas G, Thenert K, Klankermayer J, Leitner W. Angew Chem Int Ed, 2014, 53: 11010–11014
Das S, Bobbink FD, Laurenczy G, Dyson PJ. Angew Chem Int Ed, 2014, 53: 12876–12879
Zhu Q, Ma J, Kang X, Sun X, Hu J, Yang G, Han B. Sci China Chem, 2016, 59: 551–556
Santoro O, Lazreg F, Minenkov Y, Cavallo L, Cazin CSJ. Dalton Trans, 2015, 44: 18138–18144
Das S, Bobbink FD, Bulut S, Soudani M, Dyson PJ. Chem Commun, 2016, 52: 2497–2500
Blondiaux E, Pouessel J, Cantat T. Angew Chem Int Ed, 2014, 53: 12186–12190
Zheng J, Darcel C, Sortais JB. Chem Commun, 2014, 50: 14229–14232
Tlili A, Frogneux X, Blondiaux E, Cantat T. Angew Chem Int Ed, 2014, 53: 2543–2545
Cui X, Dai X, Zhang Y, Deng Y, Shi F. Chem Sci, 2014, 5: 649–655
Cui X, Zhang Y, Deng Y, Shi F. Chem Commun, 2014, 50: 13521–13524
Kon K, Siddiki SMAH, Onodera W, Shimizu K. Chem Eur J, 2014, 20: 6264–6267
Du XL, Tang G, Bao HL, Jiang Z, Zhong XH, Su DS, Wang JQ. ChemSusChem, 2015, 8: 3489–3496
Li Y, Sorribes I, Yan T, Junge K, Beller M. Angew Chem Int Ed, 2013, 52: 12156–12160
Cabrero-Antonino JR, Alberico E, Junge K, Junge H, Beller M. Chem Sci, 2016, 7: 3432–3442
Coetzee J, Dodds DL, Klankermayer J, Brosinski S, Leitner W, Slawin AMZ, Cole-Hamilton DJ. Chem Eur J, 2013, 19: 11039–11050
Cui X, Li Y, Topf C, Junge K, Beller M. Angew Chem Int Ed, 2015, 54: 10596–10599
Furst MRL, Goff RL, Quinzler D, Mecking S, Botting CH, Cole-Hamilton DJ. Green Chem, 2012, 14: 472–477
Geilen FMA, Engendahl B, Hölscher M, Klankermayer J, Leitner W. J Am Chem Soc, 2011, 133: 14349–14358
Sorribes I, Cabrero-Antonino JR, Vicent C, Junge K, Beller M. J Am Chem Soc, 2015, 137: 13580–13587
Wesselbaum S, Moha V, Meuresch M, Brosinski S, Thenert KM, Kothe J, Stein T, Englert U, Hölscher M, Klankermayer J, Leitner W. Chem Sci, 2015, 6: 693–704
Acknowledgments
This work was supported by National Natural Science Foundation of China (21603235, 21373234, 21533011), Chinese Academy of Sciences (QYZDY-SSW-SLH013) and the Recruitment Program of Global Youth Experts of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, Z., Liu, H., Qian, Q. et al. N-methylation of quinolines with CO2 and H2 catalyzed by Ru-triphos complexes. Sci. China Chem. 60, 927–933 (2017). https://doi.org/10.1007/s11426-017-9024-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-017-9024-8