Abstract
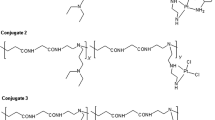
Multi-functional mikto-arm star polymers containing three different arms [hydrophilic, SN-38-P(OEGMA8–9)11, cationizable, SN-38-P(DMAEMA)38 and hydrophobic, SN-38-P(BMA)26] were prepared by RAFT polymerization via an arm-first approach using a cleavable cross-linker. The star polymers were cleaved to the linear arms with tributylphosphine as a reducing agent. The decrease in molecular weight observed is consistent with the initial stars having approximately five arms. Blue fluorescence was observed when a solution of mikto-arm star was irradiated under a 365 nm light proving the retention of the SN-38 moiety during star formation by RAFT polymerization. Thus these polymer-drug conjugates can be considered as potential delivery vehicles for cancer therapy. The P(DMAEMA) arms can be quaternized using iodomethane, allowing star polymers to bind negatively charged small interfering RNA (siRNA) and potentially be used as a carrier for that material.
Similar content being viewed by others
References
Boyer S, Bulmus V. Davis TP, Ladmiral V, Liu J, Perrier S. Bioapplications of RAFT polymerization. Chem Rev, 2009, 109: 5402–5436
Delplace V, Couvreur P, Nicolas J. Recent trends in the design of anticancer polymer prodrug nanocarriers. Polym Chem, 2014, 5: 1529–1544
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev, 2001, 47: 113–131
Wyman I, Liu G. Self-assembly and chemical processing of block copolymers: a roadmap towards a diverse array of block copolymer nanostructures. Sci China Chem, 2013, 56: 1040–1066
Jenkins AD, Jones RG, Moad G. Terminology for reversible-deactivation radical polymerization previously called “controlled” radical or “living” radical polymerization. Pure Appl Chem, 2010, 82: 483–491
Solomon DH, Rizzardo E, Cacioli P. Polymerization process and polymers produced thereby. US Patent 4581429, 1986-04-08. (Chem Abstr, 1985, 102: 221335)
George MK, Veregin RPN, Kazmaier PM, Hamer GK. Narrow molecular weight resins by a free-radical polymerization process. Macromolecules, 1993, 26: 2987–2988
Hawker CJ, Bosman AW, Harth E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev, 2001, 101: 3661–3688
Rizzardo E, Moad G. Alkoxyamine-initiated living radical polymerization: factors affecting alkoxyamine homolysis rates. Macromolecules, 1995, 28: 8722–8728
Kato M, Kamigaito M, Sawamoto M, Higashimura T. Polymerization of methyl methacrylate with the carbon tetrachloridel/dichlorotris-(triphenylphosphine)ruthedum(II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules, 1995, 28: 1721–1723
Percec V, Barboiu B. “Living” radical polymerization of styrene initiated by arenesulfonyl chlorides and CuI(bpy)nCl. Macromolecules, 1995, 28: 7970–7972
Wang JS, Matyjaszewski K. Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc, 1995, 117: 5614–5615
Ouchi M, Terashima T, Sawamoto M. Transition metal-catalyzed living radical polymerization: toward perfection in catalysis and precision polymer synthesis. Chem Rev, 2009, 109: 4963–5050
Matyjaszewski K. Atom transfer radical polymerization (ATRP): current status and future perspectives. Macromolecules, 2012, 45: 4015–4039
Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH. Living free-radical polymerization by reversible addition-fragmentation chain transfer: the RAFT process. Macromolecules, 1998, 31: 5559–5562
Moad G, Rizzardo E, Thang SH. Living radical polymerization by the RAFT process-a third update. Aust J Chem, 2012, 65: 985–1076
Moad G, Rizzardo E, Thang SH. RAFT polymerization and some of its applications. Chem Asian J, 2013, 8: 1634–1644
Chen M, Ghiggino KP, Thang SH, Wilson GJ. Star-shaped light-harvesting polymers incorporting an energy cascade. Angew chem Int Ed, 2005, 44: 4368–4372
Moad G, Chen M, Häussler M, Postma, A, Rizzardo E, Thang SH. Functional polymers for optoelectronic applications by RAFT polymerization. Polym Chem, 2011, 2: 492–519
Isakova A, Topham PD, Sutherland AJ. Controlled RAFT polymerization and Zinc binding performance of catechol-inspired homopolymers. Macromolecules, 2014, Doi: 10.1021/ma500336u
Wei Z, Hao X, Kambouris PA, Gan Z, Hughes TC. One-pot synthesis of hyperbranched polymers using small molecule and macro RAFT inimers. Polymer, 2012, 53: 1429–1436
Yang J, Luo K, Pan H, Kopeckova P, Kopecek J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic PolyHPMA conjugates. React Funct Polym, 2011, 71: 294–302
Blencowe A, Tan JF, Goh TK, Qiao GG. Core cross-linked star polymers via controlled radical polymerisation. Polymer, 2009, 50: 5–32
Gao H, Matyjaszewski K. Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linkers: from stars to gels. Prog Polym Sci, 2009, 34: 317–350
Rosselgong J, Williams EG, Le TP, Grusche F, Hinton TM, Tizard M, Gunatillake P, Thang SH. Core degradable star RAFT polymers: synthesis, polymerization, and degradation Studies. Macromolecules, 2013, 46: 9181–9188
Altintas O, Hizal G, Tunca U. ABC-type hetero-arm star terpolymers through “Click” chemistry. J Polym Sci, Part A: Polym Chem, 2006, 44: 5699–5707
Gao H, Matyjaszewski K. Synthesis of star polymers by a combination of ATRP and the “Click” coupling method. Macromolecules, 2006, 39: 4960–4965
Chan JW, Yu B, Hoyle CE, Lowe AB. Convergent synthesis of 3-arm star polymers from RAFT-prepared poly(N, N-diethylacrylamide) via a thiol-ene click reaction. Chem Commun, 2008, 4959–4961
Gao H, Matyjaszewski K. Arm-first method as a simple and general method for synthesis of miktoarm star copolymers. J Am Chem Soc, 2007, 129: 11828–11834
Cheng F, Bonder EM, Doshi A, Jäkle F. Organoboron star polymers via arm-first RAFT polymerization: synthesis, luminescent behavior, and aqueous self-assembly. Polym Chem, 2012, 3: 596–600
Shi X, Zhou W, Qiu Q, An Z. Amphiphilic heteroarm star polymer synthesized by RAFT dispersion polymerization in water/ethanol solution. Chem Commun, 2012, 48: 7389–7391
Ferreira J, Syrett J, Whittaker M, Haddleton D, Davis TP, Boyer C. Optimizing the generation of narrow polydispersity ‘arm-first’star polymers made using RAFT polymerization. Polym Chem, 2011, 2:1671–1677
Wei X, Moad G, Muir BW, Rizzardo E, Rosselgong J, Yang W, Thang SH. An arm-first approach to cleavable mikto-arm star polymers by RAFT polymerization. Macromol Rapid Commun, 2014, 35: 840–845
Rosselgong J, Armes SP, Barton W, Price D. Synthesis of highly branched methacrylic copolymers: observation of near-ideal behavior using RAFT polymerization. Macromolecules, 2009, 42: 5919–5924
Peng CL, Lai PS, Lin FH. Yueh-Hsiu Wu S, Shieh MJ. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38-loaded chlorin-core star block copolymer micelles. Biomaterials, 2009, 30: 3614–3625
Caiolfa VR, Zamai M, Fiorino A, Frigerio E, Pellizzoni C, d’Argy R, Ghiglieri A, Castelli MG, Farao M, Pesenti E, Gigli M, Angelucci F, Suarato A. Polymer-bound camptothecin: initial biodistribution and antitumour activity studies. J Control Rel, 2000, 65: 105–119
Hamaguchi T, Doi T, Eguchi-Nakajima T, Kato K, Yamada Y, Shimada Y, Fuse N, Ohtsu A, Matsumoto S, Takanashi M, Matsumura Y. Phase I study of NK012, a novel SN-38-incorporating micellar nanoparticle, in adult patients with solid tumors. Clin Cancer Res, 2010, 16: 5058–5066
Matsumura Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev, 2011, 63: 184–192
Hu X, Hu J, Tian J, Ge Z, Zhang G, Luo K, Liu S. Polyprodrug amphiphiles: hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J Am Chem Soc, 2013, 135: 17617–17629
Hu X, Tian J, Liu T, Zhang G, Liu S. Photo-triggered release of caged camptothecin prodrugs from dually responsive shell cross-linked micelles. Macromolecules, 2013, 46: 6243–6256.
Williams CC, Thang SH, Hantke T, Vogel U, Seeberger PH, Tsanaktsidis J, Lepenies B. RAFT-derived polymer-drug conjugates: poly(hydroxypropyl methacrylamide) (HPMA)-7-ethyl-10-hydroxycamptothecin (SN-38) conjugates. ChemMedChem, 2012, 7: 281–291
Moad G, Chong YK, Rizzardo E, Postma A, Thang SH. Advances in RAFT polymerization: the synthesis of polymers with defined end-groups. Polymer, 2005, 46: 8458–8468
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wei, X., Gunatillake, P.A., Moad, G. et al. Synthesis of cleavable multi-functional mikto-arm star polymer by RAFT polymerization: example of an anti-cancer drug 7-ethyl-10-hydroxycamptothecin (SN-38) as functional moiety. Sci. China Chem. 57, 995–1001 (2014). https://doi.org/10.1007/s11426-014-5128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5128-5