Abstract
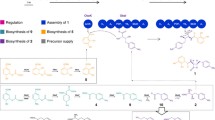
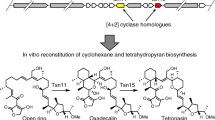
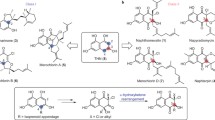
Tetronate antibiotics, a growing family of natural products featuring a characteristic tetronic acid moiety, are of importance and of particular interest for their typical structures, especially the spirotetronate structure, and corresponding versatile biological activities. Considerable efforts have persistently performed since the first tetronate was isolated, to elucidate the biosynthesis of natural tetronate products, by isotope-labeled feeding experiments, genetical characterization of biosynthetic gene clusters, and biochemical reconstitution of key enzymatic catalyzed reactions. Accordingly, the biosynthesis of spirotetronates has been gradually determined, including biosynthesis of a polyketide-derived backbone for spirotetronate aglycone, incorporation of a glycerol-derived three-carbon unit into tetronic acid moiety, formation of mature aglycone via Diels-Alder-like reaction, and decorations of aglycone with various deoxysugar moieties. In this paper, the biosynthetic investigations of natural tetronates are well documented and a common biosynthetic route for this group of natural products is summarized accordingly.
Similar content being viewed by others
References
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod, 2007, 70:461–477
Keller S, Nicholson G, Drahl C, Sorensen E, Fiedler HP, Süssmuth RD. Abyssomicins G and H and atrop-abyssomicin C from the marine Verrucosispora strain AB-18-032. J Antibiot, 2007, 60:391–394
Nakashima T, Miura M, Hara M. Tetrocarcin A inhibits mitochondrial functions of Bcl-2 and suppresses its anti-apoptotic activity. Cancer Res, 2000, 60:1229–1235
Tinhofer I, Anether G, Senfter M, Pfaller K, Bernhard D, Hara M, Greil R. Stressful death of T-ALL tumor cells after treatment with the anti-tumor agent Tetrocarcin A. FASEB J, 2002, 16:1295–1297
Bradner WT, Claridge CA, Huftalen JB. Antitumor activity of kijanimicin. J Antibiot, 1983, 36:1078–1079
Waitz JA, Horan AC, Kalyanpur M, Lee BK, Loebenberg D, Marquez JA, Miller G, Patel MG. Kijanimicin (Sch 25663), a novel antibiotic produced by Actinomadura kijaniata SCC 1256. Fermentation, isolation, characterization and biological properties. J Antibiot, 1981, 34:1101–1106
Muntwyler R, Keller-Schierlein W. Metabolic products of microorganisms. 107. Structure of chlorothricin, a new macrolide antibiotic. Helv Chim Acta, 1972, 55:2071–2094
Brufani M, Cerrini S, Fedeli W, Mazza F, Muntwyler R. Metabolic products of microorganisms. 108. Crystal structure analysis chlorothricolide methyl ester. Helv Chim Acta, 1972, 55:2094–2102
Schindler PW, Zahner H. Mode of action of the macrolide-type antibiotic, chlorothricin, kinetic study of the inhibition of pyruvate carboxylase from Bacullus stearothermophilus. Eur J Biochem, 1973, 39:591–600
Hegde VR, Patel MG, Das PR, Pramanik B, Puar MS. A family of novel macrocyclic lactones, the saccharocarcins produced by Saccharothrix aerocolonigenes subsp. antibiotica. II. Physico-chemical properties and structure determination. J Antibiot, 1997, 50:126–134
Park HR, Chijiwa S, Furihata K, Hayakawa Y, Shin-Ya K. Relative and absolute configuration of versipelostatin, a down-regulator of molecular chaperone GRP78 expression. Org Lett, 2007, 9:1457–1460
Igarashi Y, Takagi K, Kan Y, Fujii K, Harada K, Furumai T, Oki T. Arisostatins A and B, new members of tetrocarcin class of antibiotics from Micromonospora sp. TP-A0316. II. Structure determination. J Antibiot, 2000, 53:233–240
Jiang ZD, Jensen PR, Fenical W. Lobophorins A and B, new antiinflammatory macrolides produced by a tropical marine bacterium. Bioorg Med Chem Lett, 1999, 9:2003–2006
Hamaguchi T, Sudo T, Osada H. RK-682, a potent inhibitor of tyrosine phosphatase, arrested the mammalian cell cycle progression at G1phase. FEBS Lett, 1995, 372:54–58
Terui Y, Sakazaki R, Shoji J. Structures of agglomerins. J Antibiot, 1990, 43:1245–1253
Keller-Juslén C, King HD, Kuhn M, Loosli HR, Pache W, Petcher TJ, Weber HP, von Wartburg A. Tetronomycin, a novel polyether of unusual structure. J Antibiot, 1982, 35:142–150
Newbold CJ, Wallace RJ, Watt ND, Richardson AJ. Effect of the novel ionophore tetronasin (ICI 139603) on ruminal microorganisms. Appl Environ Microbiol, 1988, 54:544–547
Liu Y, Zhang S, Jung JH, Xu T. Variabilin, a chemotaxonomic marker for the family Irciniidae. Z Naturforsch C, 2007, 62:473–476
Serra T, Polónia J. Isolation of pinastric acid and ergosterol from Parmelia caperata (L.) Arch. J Pharm Sci, 1976, 65:737–738
Takeda K, Kawanishi E, Nakamura H, Yoshii E. Total synthesis of tetronolide, the aglycon of tetroncarcins. Tetrahedron Lett, 1991, 32:4925–4928
Roush WR, Scitti RJ. Enantioselective total synthesis of (−)-chloro-thricolide. J Am Chem Soc, 1994, 116:6457–6458
Jia XY, Tian ZH, Shao L, Qu XD, Zhao QF, Tang J, Tang GL, Liu W. Genetic characterization of the chlorothricin gene cluster as a model for spirotetronate antibiotic biosynthesis. Chem Biol, 2006, 13:575–585
Zhang H, White-Phillip JA, Melançon CE 3rd, Kwon HJ, Yu WL, Liu HW. Elucidation of the kijanimicin gene cluster: Insights into the biosynthesis of spirotetronate antibiotics and nitrosugars. J Am Chem Soc, 2007, 129:14670–14683
Demydchuk Y, Sun Y, Hong H, Staunton J, Spencer JB, Leadlay PF. Analysis of the tetronomycin gene cluster: Insights into the biosynthesis of a polyether tetronate antibiotic. Chembiochem, 2008, 9:1136–1145
Fang J, Zhang Y, Huang L, Jia X, Zhang Q, Zhang X, Tang G, Liu W. Cloning and characterization of the tetrocarcin A gene cluster from Micromonospora chalcea NRRL 11289 reveals a highly conserved strategy for tetronate biosynthesis in spirotetronate antibiotics. J Bacteriol, 2008, 190:6014–6125
Sun Y, Hahn F, Demydchuk Y, Chettle J, Tosin M, Osada H, Leadlay PF. In vitro reconstruction of tetronate RK-682 biosynthesis. Nat Chem Biol, 2010, 6:99–101
Gottardi EM, Krawczyk JM, von Suchodoletz H, Schadt S, Mühlenweg A, Uguru GC, Pelzer S, Fiedler HP, Bibb MJ, Stach JE, Süssmuth RD. Abyssomicin biosynthesis: Formation of an unusual polyketide, antibiotic-feeding studies and genetic analysis. Chembiochem, 2011, 12:1401–1410
He HY, Pan HX, Wu LF, Zhang BB, Chai HB, Liu W, Tang GL. Quartromicin biosynthesis: Two alternative polyketide chains produced by one polyketide synthase assembly line. Chem Biol, 2012, 19:1313–1323
Li S, Xiao J, Zhu Y, Zhang G, Yang C, Zhang H, Ma L, Zhang C. Dissecting glycosylation steps in lobophorin biosynthesis implies an iterative glycosyltransferase. Org Lett, 2013, 15:1374–1377
Kanchanabanca C, Tao WX, Hong H, Liu YJ, Hahn F, Samborskyy M, Deng ZX, Sun YH, Leadlay PF. Unusual acetylation-elimination in the formation of tetronate antibiotics. Angew Chem Int Ed, 2013, 52:5785–5788
Schobert R, Schlenk A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg Med Chem, 2008, 16:4203–4221
Holzbach R, Pape H, Hook D, Kreutzer EF, Chang C, Floss HG. Biosynthesis of the macrolide antibiotic chlorothricin: Basic building blocks. Biochemistry, 1978, 17:556–560
Mascaretti OA, Chang C, Hook D, Otsuka H, Kreuzer EF, Floss HG. Biosynthesis of the macrolide antibiotic chlorothricin. Biochemistry, 1981, 20:919–924
Tamaoki T, Tomita F. Biosynthesis of tetrocarcin. Incorporation of 14C- and 13C-labeled compounds into tetrocarcin. J Antibiot, 1983, 36:595–598
Chijiwa S, Park HR, Furihata K, Ogata M, Endo T, Kuzuyama T, Hayakawa Y, Shin-ya K. Biosynthetic studies of versipelostatin, a novel 17-membered α-tetronic acid involved macrocyclic compound isolated from Streptomyces versipellis. Tetrahedron Lett, 2003, 44:5897–5900
Sekiyama Y, Araya H, Hasumi K, Endo A, Fujimoto Y. Biosynthesis of acaterin: Incorporation of glycerol into the C3 branched unit. Tetrahedron Lett, 1998, 39:6233–6236
Mashimo Y, Sekiyama Y, Araya H, Fujimoto Y. Biosynthesis of agglomerin A: Stereospecific incorporation of pro-R- and pro-S-hydrogens at sn-C-3 of glycerol into the branched C3 moiety. Bioorg Med Chem Lett, 2004, 14:649–651
Sekiyama Y, Fujimoto Y, Hasumi K, Endo A. Biosynthesis of acaterin: Coupling of C5 unit with octanoate. J Org Chem, 2001, 66:5649–5654
Weymouth-Wilson AC. The role of carbohydrates in biologically active natural products. Nat Prod Rep, 1997, 14:99–110
Kirschning A, Bechthold FW, Rohr J. Chemical and biochemical aspects of deoxysugars and deoxysugar oligosaccharides. Top Curr hem, 1997, 188:1–84
Kaneko M, Nakashima T, Uosaki Y, Hara M, Ikeda S, Kanda Y. Synthesis of tetrocarcin derivatives with specific inhibitory activity towards Bcl-2 functions. Bioorg Med Chem Lett, 2001, 11:887–890
Biggins JB, Ternei MA, Brady SF. Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. J Am Chem Soc, 2012, 134:13192–13195
Franke J, Ishida K, Hertweck C. Genomics-driven discovery of burkholderic acid, a noncanonical, cryptic polyketide from human pathogenic Burkholderia species. Angew Chem Int Ed, 2012, 51:11611–11615
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Tao, W., Zhu, M., Deng, Z. et al. Biosynthesis of tetronate antibiotics: A growing family of natural products with broad biological activities. Sci. China Chem. 56, 1364–1371 (2013). https://doi.org/10.1007/s11426-013-4921-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-4921-x