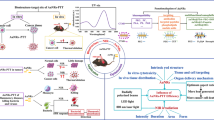
Abstract
Besides conventional surgery, radiation therapy, and chemotherapy, which all tend to have side-effects and damage normal tissues, new medical strategies, such as photothermal sensitization and photothermal ablation therapy (PTA) with near-IR laser light, have been explored for treating cancer. Much of the current excitement surrounding nanoscience is directly connected to the promise of new nanotechnology for cancer diagnosis and therapy. The basic principle behind PTA is that heat generated from light can be used to destroy cancer cells. Strong optical absorption and high efficiency of photothermal conversion at the cancer sites are critical to the success of PTA. Because of their unique optical properties, e.g., strong surface plasmon resonance (SPR) absorption, noble metal nanomaterials, such as gold and silver, have been found to significantly enhance photothermal conversion for PTA applications. Substantial effort has been made to develop metal nanostructures with optimal structural and photothermal properties. Ideal metal nanostructures should have strong and tunable SPR, be easy to deliver, have low toxicity, and be convenient for bioconjugation for actively targeting specific cancer cells. This review would highlight some gold nanostructures with various shapes and properties, including nanoparticles (NPs), nanorods (NRs), nanoshells, nanocages, and hollow nanospheres, which have been studied for PTA applications. Among these structures, hollow gold nanospheres (HGNs) exhibit arguably the best combined properties because of their small size (30–50 nm), spherical shape, and strong, narrow, and tunable SPR absorption.
Similar content being viewed by others
References
Torshina N L, Posypanova A M, Volkova A I. New sensitizers and rapid monitoring of their photodynamic activity. SPIE Proc, 1996, 2675: 254–255
Kalija O L, Meerovich G A, Torshina N L, Kogan E A, Loschenov V B, Lukyanets E A, Kogan B Y, Butenin A V, Vorozhtsov G N, Kuzmin S G, Volkova A I, Posypanova A M. Improvement of cancer PDT using sulphophthalocyanine and sodium ascorbate. SPIE Proc, 1997, 3191: 177–179
Jacques S L, McAuliffe D J. The melanosome: the threshold temperature for explosive vaporization and internal absorption coefficient during pulsed laser irradiation. Photochem Photobiol, 1991, 53: 769–775
Lin C P, Kelly N W, Sibayan S A B, Latina M A, Anderson R R. Selective cell killing by microparticle absorption of pulsed laser radiation. IEEE J Sel Top Quant, 1999, 5(4): 963–968
Lapotko D O, Lukianova E and Oraevsky A A. Selective laser nano-thermolysis of human leukemia cells with microbubbles generated around clusters of gold nanoparticles. Laser Surg Med, 2006, 38(6): 631–642
Gerstman B S, Thompson C R, Jacques S L, Rogers M E. Laser induced bubble formation in the retina. Laser Surg Med, 1996, 18(1): 10–21
West J L, Halas N J. Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics. Annu Rev Biomed Eng, 2003, 5: 285–292
Kogan B Y, Butenin A V, Torshina N L, Kogan E A, Kaliya O L, Lukyanets E A, Luzhkov Y M, Derkacheva V M, Pankratov A A, Vorozhtsov G N, Volkova A I. Aluminium sulphophthalocyanine as an NIR photosensitizer for PDT. SPIE, 1999
Meerovich G A, Torshina N L, Loschenov V B, Stratonnikov A A, Volkova A I, Vorozhtsov G N, Kaliya O L, Lukyanets E A, Kogan B Y, Butenin A V, Kogan E A, Gladskikh O P, Polyakova L N. Experimental study of PDT with aluminum sulphophthalocyanine using sodium ascorbate and hyperbaric oxygenation. SPIE, 1999
Dougherty T J. Photodynamic therapy for treatment of cancer. J Opt Soc Am B, 1984, 1(3): 555–555
Xue L Y, Chiu S M, Oleinick N L. Photodynamic therapy-induced death of MCF-7 human breast cancer cells: A role for caspase-3 in the late steps of apoptosis but not for the critical lethal event. Exp Cell Res, 2001, 263(1): 145–155
Zuluaga M F, Lange N. Combination of photodynamic therapy with anti-cancer agents. Curr Med Chem, 2008, 15(17): 1655–1673
Thomsen S. Pathological analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem Photobiol, 1991, 53(6): 825–835
Balogh L P, Tse C, Lesniak W, Ye J, O’Donnell M, Khan M K. Photomechanical therapy: destruction of nanocomposite labeled cells by laser induced optical breakdown. Nanomed-Nanotech Bio Med, 2007, 3(4): 350–350
Hirsch L R, Stafford R J, Bankson J A, Sershen S R, Rivera B, Price R E, Hazle J D, Halas N J, West J L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. P Natl Acad Sci USA, 2003, 100(23): 13549–13554
Loo C, Lin A, Hirsch L, Lee M H, Barton J, Halas N, West J, Drezek R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Tech Cancer Res Tr, 2004, 3(1): 33–40
ONeal D P, Hirsch L R, Halas N J, Payne J D, West J L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett, 2004, 209(2): 171–176
Camerin M, Rello S, Villanueva A, Ping X Z, Kenney M E, Rodgers M A J, Jori G. Photothermal sensitisation as a novel therapeutic approach for tumours: Studies at the cellular and animal level. Eur J Cancer, 2005, 41(8): 1203–1212
Gobin A M, Lee M H, Halas N J, James W D, Drezek R A, West J L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett, 2007, 7(7): 1929–1934
Ji X J, Shao R P, Elliott A M, Stafford R J, Esparza-Coss E, Bankson J A, Liang G, Luo Z P, Park K, Markert J T, Li C. Bifunctional gold nanoshells with a superparamagnetic iron oxide-silica core suitable for both MR imaging and photothermal therapy. J Phys Chem C, 2007, 111(17): 6245–6251
Skrabalak S E, Au L, Lu X M, Li X D, Xia Y N. Gold nanocages for cancer detection and treatment. Nanomedicine, 2007, 2(5): 657–668
Huang X H, El-Sayed I H, Qian W, El-Sayed M A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: A potential cancer diagnostic marker. Nano Lett, 2007, 7(6): 1591–1597
Melancon M P, Lu W, Yang Z, Zhang R, Cheng Z, Elliot A M, Stafford J, Olson T, Zhang J Z, Li C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Therapeut, 2008, 7(6): 1730–1739
Lu W, Xiong C Y, Zhang G D, Huang Q, Zhang R, Zhang J Z, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin Cancer Res, 2009, 15(3): 876–886
Paltauf G, Dyer P E. Photomechanical processes and effects in ablation. Chem Rev, 2003, 103(2): 487–518
Jain P K, Huang X H, El-Sayed I H, El-Sayed M A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Accounts Chem Res, 2008, 41(12): 1578–1586
Chakravarty P, Marches R, Zimmerman N S, Swafford A D E, Bajaj P, Musselman I H, Pantano P, Draper R K, Vitetta E S. Thermal ablation of tumor cells with anti body-functionalized single-walled carbon nanotubes. P Natl Acad Sci USA, 2008, 105(25): 8697–8702
Arayne M S, Sultana N. Nanoparticles in drug delivery for the treatment of cancer. Pak J Pharm Sci, 19(3): 2006, 258–268
Ambrogi M C, Fontanini G, Cioni R, Faviana P, Fanucchi O, Mussi A. Biologic effects of radiofrequency thermal ablation on non-small cell lung cancer: Results of a pilot study. J Thorac Cardiov Sur, 2006, 131(5): 1002–1006
Stern J M, Stanfield J, Kabbani W, Hsieh J T, Cadeddu J R A. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urology, 2008, 179(2): 748–753
Boaz T L, Lewin J S, Chung Y C, Duerk J L, Clampitt M E, Haaga J R. MR monitoring of MR-guided radiofrequency thermal ablation of normal liver in an animal model. J Magn Reson Imaging, 1998, 8(1): 64–69
Nguyen C T, Campbell S C. Salvage of local recurrence after primary thermal ablation for small renal masses. Exp Rev Anticancer Ther, 2008, 8(12): 1899–1905
Schwartzberg A M, Grant C D, van Buuren T, Zhang J Z. Reduction of HAuCl4 by Na2S revisited: The case for Au nanoparticle aggregates and against Au2S/Au Core/Shell particles. J Phys Chem C, 2007, 111(25): 8892–8901
Schwartzberg A M, Zhang J Z. Novel optical properties and emerging applications of metal nanostructures. J Phys Chem C, 2008, 112: 10323–10337
Zhang J Z, Noguez C. Plasmonic optical properties and applications of metal nanomaterials. Plasmonics, 2008, 3(4): 127–150
Chithrani B D, Ghazani A A, Chan W C W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett, 2006, 6(4): 662–668
Schwartzberg A M, Olson T Y, Talley C E, Zhang J Z. Synthesis, characterization, and tunable optical properties of hollow gold nanospheres. J Phys Chem B, 2006, 110(40): 19935–19944
Bowler K, Laudien H, Laudien I. Cellular heat injury. J Therm Biol, 1983, 8(4): 426–430
Bass H, Coakley W T, Moore J L, Tilley D. Hyperthermia-induced changes in the morphology of CHO-K1 and their refractile inclusions. J Therm Biol, 1982, 7(4): 231–242
Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag P M. Hyperthermia in combined treatment of cancer. Lancet Oncol, 2002, 3(8): 487–497
Tong L, Zhao Y, Huff T B, Hansen M N, Wei A, Cheng J X. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv Mater, 2007, 19(20): 3136–3141
Kotaidis V, Plech A. Cavitation dynamics on the nanoscale. Appl Phys Lett, 2005, 87(21): 213102–213104
Zharov V P, Galitovskaya E N, Johnson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: Potential for cancer therapy. Laser Surg Med, 2005, 37(3): 219–226
Letfullin R R, Joenathan C, George T F, Zharov V P. Laser-induced explosion of gold nanoparticles: potential role for nanophotothermolysis of cancer. Nanomedicine, 2006, 1(4): 473–480
Huang X, Qian W, El-Sayed I H, El-Sayed M A. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Laser Surg Med, 2007, 39(9): 747–753
Norman T J, Grant C D, Magana D, Zhang J Z, Liu J, Cao D L, Bridges F, van Buuren A. Near infrared optical absorption of gold nanoparticle aggregates. J Phys Chem B, 2002, 106(28): 7005–7012
Grant C D, Schwartzberg A M, Norman T J, Zhang J Z. Ultrafast electronic relaxation and coherent vibrational oscillation of strongly coupled gold nanoparticle aggregates. J Am Chem Soc, 2003, 125(2): 549–553
Akiyama Y, Mori T, Katayama Y, Miidome T. The effects of PEG grafting level and injection dose on gold nanorod biodistribution in the tumor-bearing mice. J Control Release, 2009, 139(1): 81–84
Didychuk C L, Ephrat P, Chamson-Reig A, Jacques S L, Carson J J L. Depth of photothermal conversion of gold nanorods embedded in a tissue-like phantom. Nanotechnology, 2009, 20(19): 151102–151110
Huang Y F, Sefah K, Bamrungsap S, Chang H T, Tan W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir, 2008, 24(20): 11860–11865
von Maltzahn G, Park J H, Agrawal A, Bandaru N K, Das S K, Sailor M J, Bhatia S N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res, 2009, 69(9): 3892–3900
Goodrich G P, Payne J D, Sharp K, Bao L, Sang K L. Efficacy of photothermal ablation using intravenously delivered NIR-absorbing nanorods in colon cancer. in Energy-based Treatment of Tissue and Assessment, SPIE, 2009
Huang X H, El-Sayed I H, Qian W, El-Sayed M A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc, 2006, 128(6): 2115–2120
Wang C G, Chen J, Talavage T, Irudayaraj J. Gold nanorod/Fe3O4 nanoparticle “nano-pearl-necklaces” for simultaneous targeting, dual- mode imaging, and photothermal ablation of cancer cells. Angew Chem-Int Edit, 2009, 48(15): 2759–2763
Lal S, Clare S E, Halas N J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Accounts Chem Res, 2008, 41(12): 1842–1851
Hao E, Li S Y, Bailey R C, Zou S L, Schatz G C, Hupp J T. Optical properties of metal nanoshells. J Phys Chem B, 2004, 108(4): 1224–1229
Zhang J Z, Schwartzberg A M, Norman T, Grant C D, Liu J, Bridges F, van Buuren T. Comment on “gold nanoshells improve single nanoparticle molecular sensors”. Nano Lett, 2005, 5(4): 809–810
Zhang J Z, Wang Z L, Liu J, Chen S, Liu G-y. Self-assembled Nanostructures. Nanoscale Science and Technology. New York: Kluwer Academic/Plenum Publishers, 2003. 316
Zhang J Z. Optical Properties and Spectroscopy of Nanomaterials. Singapore: World Scientific Publisher, 2009. 383
Schwartz J A, Shetty A M, Price R E, Stafford R J, Wang J C, Uthamanthil R K, Pham K, McNichols R J, Coleman C L, Payne J D. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res, 2009, 69(4): 1659–1667
Gobin A M, Moon J J and West J L. EphrinA1-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomed, 2008, 3(3): 351–358
Lowery A R, Gobin A M, Day E S, Halas N J, West J L. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomed, 2006, 1(2): 149–154
Bernardi R J, Lowery A R, Thompson P A, Blaney S M, West J L. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J Neuro-Oncol, 2008, 86(2): 165–172
Male K B, Lachance B, Hrapovic S, Sunahara G, Luong J H T. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal Chem, 2008, 80(14): 5487–5493
Hauck T S, Ghazani A A, Chan W C W. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small, 2008, 4(1): 153–159
Parab H J, Chen H M, Lai T C, Huang J H, Chen P H, Liu R S, Hsiao M, Chen C H, Tsai D P, Hwu Y K. Biosensing, cytotoxicity, and cellular uptake studies of surface-modified gold nanorods. J Phys Chem C, 2009, 113(18): 7574–7578
Alkilany A M, Nagaria P K, Hexel C R, Shaw T J, Murphy C J, Wyatt M D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small, 2009, 5(6): 701–708
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the US NSF, US DoD, and NASA UARC
Rights and permissions
About this article
Cite this article
Rozanova, N., Zhang, J. Photothermal ablation therapy for cancer based on metal nanostructures. Sci. China Ser. B-Chem. 52, 1559–1575 (2009). https://doi.org/10.1007/s11426-009-0247-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0247-0