Abstract
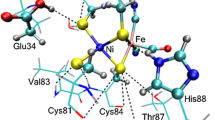
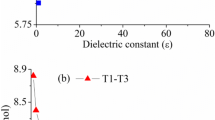
A representative acetate-(5-methylimidazole)-methanol system has been employed as a model of catalytic triad in serine protease to validate the formation processes of low-barrier H-bonds (LBHB) at the B3LYP/6-311++G** level of theory, and variable H-bonding characters from conventional ones to LBHBs have been represented along with the proceedings of proton transfer. Solvent effect is an important factor in modulation of the existence of an LBHB, where an LBHB (or a conventional H-bond) in the gas phase can be changed into a non-LBHB (an LBHB) upon solvation. The origin of the additional stabilization energy arising from the LBHB may be attributed to the H-bonding energy difference before and after proton transfer because the shared proton can freely move between the proton donor and proton acceptor. Most importantly, the order of magnitude of the stabilization energy depends on the studied systems. Furthermore, the nonexistence of LBHBs in the catalytic triad of serine proteases has been verified in a more sophisticated model treated using the ONIOM method. As a result, only the single proton transfer mechanism in the catalytic triad has been confirmed and the origin of the powerful catalytic efficiency of serine proteases should be attributed to other factors rather than the LBHB.
Similar content being viewed by others
References
Polgár L, Halász P. Current problems in mechanistic studies of serine and cysteine proteinases. Biochem J, 1982, 207: 1–10
Westler W M, Weinhold F, Markley J L. Quantum chemical calculations on structural models of the catalytic site of Chymotrypsin: comparison of calculated results with experimental data from NMR spectroscopy. J Am Chem Soc, 2002, 124(48): 14373–14381
Hedstrom L. Serine protease mechanism and specificity. Chem Rev, 2002, 102(12): 4501–4523
Pan Y, McAllister M A. Characterization of low-barrier hydrogen bonds. 5. Microsolvation of enol-enolate. An ab initio and DFT investigation. J Org Chem, 1997, 62(23): 8171–8176
Frey P A, Whitt S A, Tobin J B. A low-barrier hydrogen bond in the catalytic triad of serine proteases. Science, 1994, 264(5167): 1927–1930
Garcia-Viloca M, Gonzalez-Lafont A, Lluch J M. On pK a matching as a requirement to form a low-barrier hydrogen bond. A theoretical study in gas phase. J Phys Chem A, 1997, 101(21): 3880–3886
Pan Y, McAllister M A. Characterization of low-barrier hydrogen bonds. 6. Cavity polarity effects on the formic acid-formate anion model system. An ab initio and DFT investigation. J Am Chem Soc, 1998, 120(1): 166–169
Cleland W W, Kreevoy M M. Low-barrier hydrogen bonds and enzymic catalysis. Science, 1994, 264(5167): 1887–1890
Schiott B, Iversen B B, Madsen G K H, Larsen F K, Bruice T C. On the electronic nature of low-barrier hydrogen bonds in enzymatic reactions. Proc Natl Acad Sci U. S. A, 1998, 95(22): 12799–12802
Harris T K, Mildvan A S. High-precision measurement of hydrogen bond lengths in proteins by nuclear magnetic resonance methods. Proteins, 1999, 35(3): 275–282
Ishida T. Low-barrier hydrogen bond hypothesis in the catalytic triad residue of serine proteases: Correlation between structural rear-rangement and chemical shifts in the acylation process. Biochemistry, 2006, 45(17): 5413–5420
Fuhrmann C N, Daugherty M D, Agard D A. Subangstrom crystallography reveals that short ionic hydrogen bonds, and not a His-Asp low-barrier hydrogen bond, stabilize the transition state in serine protease catalysis. J Am Chem Soc, 2006, 128(28): 9086–9102
Liptak M D, Gross K C, Seybold P G, Feldgus S, Shields, G C. Absolute pK a determinations for substituted phenols. J Am Chem Soc, 2002, 124(22): 6421–6427
Chen J, McAllister M A, Lee J K, Houk K N. Short, strong hydrogen bonds in the gas phase and in solution: theoretical exploration of pK a matching and environmental effects on the strengths of hydrogen bonds and their potential roles in enzymatic catalysis. J Org Chem, 1998, 63(14): 4611–4619
Kim Y, Ahn K. Theoretical study of the role of low-barrier hydrogen bonds in enzyme catalysis: a model of proton transfer in serine protease. Theor Chem Acc, 2001, 106(3): 171–177
Tobin J B, Whitt S A, Cassidy C S. Frey P A. Low-barrier hydrogen bonding in molecular complexes analogous to histidine and aspartate in the catalytic triad of serine proteases. Biochemistry, 1995, 34(21): 6919–6924
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 20633060 & 20573063), the Natural Science Foundation of Shandong Province (Grant No. Y2007B23), the Scientific Research Foundation of Qufu Normal University (Grant Nos. Bsqd2007003 and Bsqd2007008), and the State Key Laboratory of Physical Chemistry of Solid Surfaces (Xiamen University)
Rights and permissions
About this article
Cite this article
Li, P., Wang, W., Bi, S. et al. Theoretical studies of the proton transfer behaviors in molecular complexes analogous to catalytic triad of serine protease: Toward understanding the existence and significance of the low-barrier hydrogen-bond in enzymatic catalysis. Sci. China Ser. B-Chem. 52, 131–136 (2009). https://doi.org/10.1007/s11426-009-0022-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0022-2