Abstract
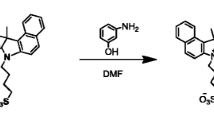
A fluorogen named 1-decyl-1-methyl-2,5-bis{4-[(N,N-diethylamino)methyl]phenyl}-3,4-diphenylsilole (3) was synthesized. It emitted weakly as isolated molecule but strongly as supramolecular aggregate, showing a characteristic behavior of aggregation-induced emission (AIE). The molecules of 3 formed highly emissive nanoparticles in aqueous media, which quickly and selectively marked cytoplasm of HeLa cells and posed no toxicity to the living cells. The fluorogen is thus a promising candidate material for cell imaging as a sensitive, selective and cytocompatible biosensor.
Similar content being viewed by others
References
Wang Y, Shyy J Y J, Chien S. Fluorescence proteins, live-cell imaging, and mechanobiology: Seeing is believing. Ann Rev Biomed Eng, 2008, 10: 1–38
Van Engelenburg S B, Palmer A E. Fluorescent biosensors of protein function. Curr Opin Chem. Biol. 2008, 12: 60–65
Borisov S M, Wolfbeis O S. Optical biosensors. Chem Rev, 2008, 108: 423–461
Domaille D W, Que E L, Chang C J. Synthetic fluorescent sensors for studying the cell biology of metals. Nat Chem Biol, 2008, 4: 168–175
Quina F H, Lissi E A. Photoprocesses in microaggregates. Acc Chem Res, 2004, 37: 703–710
Capek I. Fate of excited probes in micellar systems. Adv Coll Interf Sci, 2002, 97: 91–149
Michalet X, Pinaud F F, Bentolila L A, Tsay J M, S. Doose, Li J J, Sundaresan G, Wu A M, Gambhir S S, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science, 2005, 307: 538–544
Medintz I L, Uyeda H T, Goldman E R, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater, 2005, 4: 435–446
Li H C, Zhou Q F, Liu W, Yan B, Zhao Y B, Jiang G B. Progress in the toxicological researches for quantum dots. Sci China Ser B-Chem, 2008, 51: 393–400
Burns A, Ow H, Wiesner U. Fluorescent core-shell silica nanoparticles: Towards “Lab on a Particle” architectures for nanobiotechnology. Chem Soc Rev, 2006,35: 1028–042
Krebs F C, Miller S R, Catalone B J, Fichorova R, Anderson D, Malamud D, Howett M K, Wigdahl B. Comparative in vitro sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfate. Antimicrob Agents Chemother, 2002, 46: 2292–2298
Luo J D, Xie Z L, Lam J W Y, Cheng L, Chen H Y, Qiu C F, Kwok H S, Zhan X W, Liu Y Q, Zhu D B, Tang B Z. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun, 2001, 1740–1741
Yu G, Yin S W, Liu Y Q, Chen J S, Xu X J, Sun X B, Ma D G, Zhan X W, Peng Q, Shuai Z G, Tang B Z, Zhu D B, Fang W H, Luo Y. Structures, electronic states, photoluminescence, and carrier transport properties of 1,1-disubstituted 2,3,4,5-tetraphenylsiloles. J Am Chem Soc, 2005, 127(17): 6335–6346
Zeng Q, Li Z, Dong Y Q, Di C A, Qin A J, Hong Y N, Ji L, Zhu Z C, Jim C K W, Yu G, Li Q Q, Li Z G, Liu Y Q, Qin J G, Tang B Z. Fluorescence enhancements of benzene-cored luminophors by restricted intramolecular rotations: AIE and AIEE effects. Chem Commun, 2007, 70–72
Dong Y Q, Lam J W Y, Qin A J, Li Z, Sun J Z, Sung H H Y, Williams I D, Tang B Z. Switching the light emission of (4-biphenylyl)phenyldibenzofulvene by morphological modulation: Crystallization-induced emission enhancement. Chem Commun, 2007, 40–42
Dong Y Q, Lam J W Y, Qin A J, Li Z, Liu J Z, Sun J Z, Dong Y P, Tang B Z. Endowing hexaphenylsilole with chemical sensory and biological probing properties by attaching amino pendants to the silolyl core. Chem Phys Lett, 2007, 446: 124–127
Hong Y N, Häuβler M, Lam J W Y, Li Z, Sin K K, Dong Y Q, Tong H, Liu J Z, Qin A J, Renneberg R, Tang B Z. Label-free fluorescent probing of G-quadruplex formation and real-time monitoring of DNA folding by a quaternized tetraphenylethene salt with aggregationinduced emission characteristics. Chem Eur J, 2008, 14: 6428–6437
Chen J, Law C C W, Lam J W Y, Dong Y, Lo S M F, Williams I D, Zhu D, Tang B Z. Synthesis, light emission, nanoaggregation, and restricted intramolecular rotation of 1,1-substituted 2,3,4,5-tetra- phenylsiloles. Chem Mater, 2003, 15: 1535–1546
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the Research Grants Council of Hong Kong (Grant Nos. 603008, 601608 and 602707), the National Natural Science Foundation of China (Grant No. 20634020) and the CAO GuangBiao Foundation of Zhejiang University.
Rights and permissions
About this article
Cite this article
Yu, Y., Hong, Y., Feng, C. et al. Synthesis of an AIE-active fluorogen and its application in cell imaging. Sci. China Ser. B-Chem. 52, 15–19 (2009). https://doi.org/10.1007/s11426-009-0008-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0008-0