Abstract
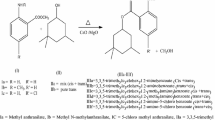
A novel heterogeneous strong acid catalyst was synthesized through the copolymerization of p-toluenesulfonic acid and paraformaldehyde and utilized for the synthesis of fructone. The results showed that the catalyst was very efficient for the reaction with the yield over 95%. The advantages of extremely high density of acidity, high thermal and chemical stability, low cost for the simple synthetic procedure, and reusability made the catalyst one of the best choices for the reaction.
Similar content being viewed by others
References
Anastas P T, Kirchhoff M M. Origins, current status, and future challenges of green chemistry. Acc Chem Res, 2002, 35: 685–694
DeSimone J M. Practical approaches to green solvents. Science, 2002, 297: 799–803
Harton B. Green chemistry puts down roots. Nature, 1999, 400(19):797–799
Anastas P T, Zimmermann J B. Design through the 12 principles of green engineering. Environ Sci Technol, 2003, 37: 94A–101A
Clark J H. Solid acids for green chemistry. Acc Chem Res, 2002, 35:791–797
Misono M, Acad C R. Heterogeneous catalysis. Sci Ser Iic, 2000, 3:471–475
Okuhara T. Water-tolerant solid acid catalysts. Chem Rev, 2002, 102(10): 3641–3666
Smith K, El-Hiti G A, Gamal A, Jayne A J, Butters M. Acetylation of aromatic ethers using acetic anhydride over solid acid catalysts in a solvent-free system. Scope of the reaction for substituted ethers. Org Biomol Chem, 2003, 1: 1560–1564
Bauer K, Garbe D, Surburg H. Common Fragrances and Flavors Materials. 2nd Ed. New York: VCH, 1990
Li D, Shi F, Peng J, Guo S, Deng Y. Application of functional ionic liquids possessing two adjacent acid sites for acetalization of aldehydes. J Org Chem, 2004, 69(10): 3582–3585
Wu H H, Yang F, Cui P, Tang J, He M Y. An efficient procedure for protection of carbonyls in Brønsted acidic ionic liquid [Hmim]BF4. Tetrahedron Lett, 2004, 45(25): 4963–4965
Greene T W, Wuts P G M. Protective Groups in Organic Synthesis. New York: John Wiley and Sons, 1999. 293
Katritzky A R, Otto M C. Comprehensive Organic Functional Group Transformations. Oxford: Pergamon Press, 1995. Vol. 4, 176
Kocienski P J. Protecting Groups. New York: George Thieme Verlag, 1994. 156
Clode D M. Carbohydrate cyclic acetal formation and migration. Chem Rev, 1979, 79(6): 491–513
Climent M J, Corma A, Veltyl A, Susartey M, Zeolites for the production of fine chemicals: Synthesis of the fructone fragrancy. J Catal, 2000, 196: 345–351
Wadlinger R L, Kerr G T, Rosinski E. US Patent 3 308 069, 1967-03-07
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Key Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (Grant No. 2006BAE03B06)
Rights and permissions
About this article
Cite this article
Gao, S., Liang, X., Yang, J. et al. Highly efficient heterogeneous procedure for the synthesis of fructone fragrancy. Sci. China Ser. B-Chem. 51, 646–650 (2008). https://doi.org/10.1007/s11426-008-0065-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0065-9