Abstract
Purpose
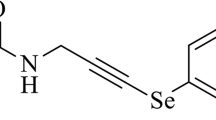
N-Benzyl-substituted phenethylamines (NBOMes) are psychoactive drugs, which induce various symptoms like serotonin syndrome, even at low doses. Recently, we reported the first lethal case of a designer drug, 2-(4-bromo-2, 5-dimethoxyphenyl)-NBOMe (25B-NBOMe) intoxication with severe rhabdomyolysis, evaluated by clinical, pathological, and toxicological analyses. We also confirmed that 25B-NBOMe can induce rhabdomyolysis using a zebrafish model. To further elucidate pathomechanism of NBOMes-induced rhabdomyolysis, we treated zebrafish with a similar designer drug, 2-(4-methyl-2, 5-dimethoxyphenyl)-NBOMe (25D-NBOMe).
Methods
Zebrafish treated with a designer drug, 25D-NBOMe, were examined survival rate and were analyzed skeletal muscle degeneration by birefringence. They were also analyzed expression levels of 5-HT2A and 5-HT2C receptors and ryanodine receptor.
Results
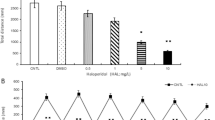
The 25D-NBOMe-treated fish showed decreased survival rate and skeletal muscle degeneration detected by birefringence mimicking to those of 25B-NBOMe-treated fish. We revealed that 25D-NBOMe induced up-regulation of the expression of 5-HT2A and 5-HT2C receptors, and rhabdomyolysis was inhibited by the 5-HT2A receptor antagonist, aripiprazole and 5-HT2C receptor antagonist, SB242084. Moreover, ryanodine receptor expression was up-regulated in 25D-NBOMe-treated fish as compared to that of untreated fish, and the up-regulated expression of ryanodine receptor in 25D-NBOMe-treated fish was improved with co-treatment aripiprazole and SB242084.
Conclusions
Our findings suggest that multiple 5-HT receptors have important roles in NBOMes-induced rhabdomyolysis together with other serotonin-related symptoms. Zebrafish is a highly useful model for therapeutic studies of rhabdomyolysis induced by psychoactive designer drugs.




Similar content being viewed by others
References
Kyriakou C, Marinelli E, Frati P, Santurro A, Afxentiou M, Zaami S, Busardo FP (2015) NBOMe: new potent hallucinogens–pharmacology, analytical methods, toxicities, fatalities: a review. Eur Rev Med Pharmacol Sci 19:3270–3281 (PMID: 26400534)
Werneke U, Jamshidi F, Taylor DM, Ott M (2016) Conundrums in neurology: diagnosing serotonin syndrome—a meta-analysis of cases. BMC Neurol 16:97. https://doi.org/10.1186/s12883-016-0616-1(open access article)
Boyer EW, Shannon M (2005) The serotonin syndrome. N Engl J Med 352:1112–1120. https://doi.org/10.1056/NEJMra041867
Hill SL, Doris T, Gurung S, Katebe S, Lomas A, Dunn M, Blain P, Thomas SH (2013) Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clin Toxicol 51:487–492. https://doi.org/10.3109/15563650.2013.802795
Suzuki J, Dekker MA, Valenti ES, Arbelo Cruz FA, Correa AM, Poklis JL, Poklis A (2015) Toxicities associated with NBOMe ingestion—a novel class of potenthallucinogens: a review of the literature. Psychosomatics 56:129–139. https://doi.org/10.1016/j.psym.2014.11.002
Yoshida K, Saka K, Shintani-Ishida K, Maeda M, Nakajima N, Hara S, Ueno M, Sasaki M, Iwase H, Sakamoto T (2015) A case of fatal intoxication due to the new designer drug 25B-NBOMe. Forensic Toxicol 33:396–401. https://doi.org/10.1007/s11419-015-0276-7
Tang MH, Ching CK, Tsui MS, Chu FK, Mak TW (2014) Two cases of severe intoxication associated with analytically confirmed use of the novel psychoactive substances 25B-NBOMe and 25C-NBOMe. Clin Toxicol 52:561–565. https://doi.org/10.3109/15563650.2014.909932
Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP (2007) Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT) 2A and 5-HT 2C receptors. J Pharmacol Exp Ther 321:1054–1061. https://doi.org/10.1124/jpet.106.117507
Nichols DE (2004) Hallucinogens. Pharmacol Ther 101:131–181. https://doi.org/10.1016/j.pharmthera.2003.11.002
Hajduch E, Dombrowski L, Darakhshan F, Rencurel F, Marette A, Hundal HS (1999) Biochemical localisation of the 5-HT2A (serotonin) receptor in rat skeletal muscle. Biochem Biophys Res Commun 257:369–372. https://doi.org/10.1006/bbrc.1999.0471
Schneider H, Fritzky L, Williams J, Heumann C, Yochum M, Pattar K, Noppert G, Mock V, Hawley E (2012) Cloning and expression of a zebrafish 5-HT(2C) receptor gene. Gene 502:108–117. https://doi.org/10.1016/j.gene.2012.03.070
Hajduch E, Rencurel F, Balendran A, Batty IH, Downes CP, Hundal HS (1999) Serotonin (5-hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle. J Biol Chem 274:13563–13568. https://doi.org/10.1074/jbc.274.19.13563
Bassett DI, Currie PD (2003) The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum Mol Genet 12:265–270. https://doi.org/10.1093/hmg/ddg279
Guyon JR, Goswami J, Jun SJ, Thorne M, Howell M, Pusack T, Kawahara G, Steffen LS, Galdzicki M, Kunkel LM (2009) Genetic isolation and characterization of a splicing mutant of zebrafish dystrophin. Hum Mol Genet 18:202–211. https://doi.org/10.1093/hmg/ddn337
Kawahara G, Guyon JR, Nakamura Y, Kunkel LM (2010) Zebrafish models for human FKRP muscular dystrophies. Hum Mol Genet 19:623–633. https://doi.org/10.1093/hmg/ddp528
Siebel AM, Vianna MR, Bonan CD (2014) Pharmacological and toxicological effects of lithium in zebrafish. ACS Chem Neurosci 5:468–476. https://doi.org/10.1021/cn500046
Kawahara G, Karpf JA, Myers JA, Alexander MS, Guyon JR, Kunkel LM (2011) Drug screening in a zebrafish model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 108:5331–5336. https://doi.org/10.1073/pnas.1102116108
Waugh TA, Horstick E, Hur J, Jackson SW, Davidson AE, Li X, Dowling JJ (2014) Fluoxetine prevents dystrophic changes in a zebrafish model of Duchenne muscular dystrophy. Hum Mol Genet 23:4651–4662. https://doi.org/10.1093/hmg/ddu185(open access article)
Kawahara G, Maeda H, Kikura-Hanajiri R, Yoshida KI, Hayashi YK (2017) The psychoactive drug 25B-NBOMe recapitulates rhabdomyolysis in zebrafish larvae. Forensic Toxicol 35:369–375. https://doi.org/10.1007/s11419-017-0366-9(open access article)
Nüsslein-Volhard C, Dahm R (eds) (2002) Zebrafish: a practical approach. Oxford University Press, Oxford. https://doi.org/10.1017/s00166723032/6384
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. https://doi.org/10.1002/aja.1002030302
Pytliak M, Vargová V, Mechírová V, Felšöci M (2011) Serotonin receptors—from molecular biology to clinical applications. Physiol Res 60:15–25 (PMID: 20945968)
Stewart AM, Cachat J, Gaikwad S, Robinson KS, Gebhardt M, Kalueff AV (2013) Perspectives on experimental models of serotonin syndrome in zebrafish. Neurochem Int 62:893–902. https://doi.org/10.1016/j.neuint.2013.02.018
Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (2014) Synthesis and structure-activity relationships of N-benzylphenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci 5:243–249. https://doi.org/10.1021/cn400216u
Casale JF, Hays PA (2012) Characterization of eleven 2,5-dimethoxy-N-(2-methoxybenzyl) phenethylamine (NBOMe) derivatives and differentiation from their 3- and 4-methoxybenzyl analogues—part I. Microgram J 9:84–109 (open access article)
Morton RA, Baptista-Hon DT, Hales TG, Lovinger DM (2015) Agonist- and antagonist-induced up-regulation of surface 5-HT3A receptors. Br J Pharmacol 172:4066–4077. https://doi.org/10.1111/bph.13197
Bencherif M, Fowler K, Lukas RJ, Lippiello PM (1995) Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. J Pharmacol Exp Ther 275:987–994 (PMID: 7473192)
Franklin JM, Carrasco GA (2013) G-protein receptor kinase 5 regulates the cannabinoid receptor 2-induced up-regulation of serotonin 2A receptors. J Biol Chem 288:15712–15724. https://doi.org/10.1074/jbc.M113.454843
Guillet-Deniau I, Burnol AF, Girard J (1997) Identification and localization of a skeletal muscle secrotonin 5-HT2A receptor coupled to the Jak/STAT pathway. J Biol Chem 272:14825–14829. https://doi.org/10.1074/jbc.272.23.14825(open access article)
Gatmaitan Z, Varticovski L, Ling L, Mikkelsen R, Steffan AM, Arias IM (1996) Studies on fenestral contraction in rat liver endothelial cells in culture. Am J Pathol 148:2027–2041 (PMC:1861643)
Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD (1996) Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380:72–75. https://doi.org/10.1038/380072a0
Galindo L, Moreno E, López-Armenta F, Guinart D, Cuenca-Royo A, Izquierdo-Serra M, Xicota L, Fernandez C, Menoyo E, Fernández-Fernández JM, Benítez-King G, Canela EI, Casadó V, Pérez V, de la Torre R, Robledo P (2018) Cannabis users show enhanced expression of CB1-5HT2A receptor heteromers in olfactory neuroepithelium cells. Mol Neurobiol 55:6347–6361. https://doi.org/10.1007/s12035-017-0833-7
Xiang M, Jiang Y, Hu Z, Yang Y, Botchway BOA, Fang M (2017) Stimulation of anxiety-like behavior via ERK pathway by competitive serotonin receptors 2A and 1A in post-traumatic stress disordered mice. Neurosignals 25:39–53. https://doi.org/10.1159/000481791(open access article)
Amodeo DA, Rivera E, Cook EH Jr, Sweeney JA, Ragozzino ME (2017) 5HT2A receptor blockade in dorsomedial striatum reduces repetitive behaviors in BTBR mice. Genes Brain Behav 16:342–351. https://doi.org/10.1111/gbb.12343
Amidfar M, Kim YK, Colic L, Arbabi M, Mobaraki G, Hassanzadeh G, Walter M (2017) Increased levels of 5HT2A receptor mRNA expression in peripheral blood mononuclear cells of patients with major depression: correlations with severity and duration of illness. Nord J Psychiatry 71:282–288. https://doi.org/10.1080/08039488.2016.1276624
Acknowledgements
This work was supported partly by a Grant-in-Aid for Challenging Exploratory Research, Scientific Research (S) (B) and (C) from the Japan Society for the Promotion of Science (Grant nos. 18K19703, 16K09218, 17H06112, 16H05274), by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (Grant no. 5020-03), by Research on Rare and Intractable Diseases, Health and Labor Science Research (Grant no. H26-nanchitou(nan)-ippan-079), and by Intramural Research Grant for Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry (29-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Ethical approval
The use of zebrafish for this study was approved by the Experimental Animal Committee of Tokyo Medical University (approval number: H290067). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawahara, G., Nakayashiki, M.S., Maeda, H. et al. Antagonists for serotonin receptors ameliorate rhabdomyolysis induced by 25D-NBOMe, a psychoactive designer drug. Forensic Toxicol 38, 122–128 (2020). https://doi.org/10.1007/s11419-019-00495-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-019-00495-w