Abstract
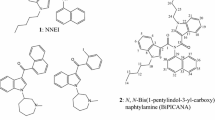
During our careful surveillance of unregulated drugs in January to February 2011, we found two new compounds used as adulterants in herbal products obtained via the Internet. These compounds were identified by liquid chromatography–mass spectrometry, gas chromatography-mass spectrometry, accurate mass spectrometry, and nuclear magnetic resonance spectroscopy. The first compound identified was a benzoylindole (2-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone (1), which is a positional isomer of (4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone (RCS-4, 4). The second compound was 1-(5-fluoropentyl-1H-indol-3-yl)-(naphthalene-1-yl)methanone (AM-2201, 2). The compound 2 has been reported to be a cannabinoid receptor agonist. Because the cannabimimetic effects of compounds 1 and 4 have not been reported to date, their biological activities were evaluated by measuring the activation of [35S] guanosine-5′-O-(3-thio)-triphosphate binding to guanine nucleotide-binding proteins, together with those of other synthetic cannabimimetic compounds. For quantitation of the above two compounds (1 and 2) and previously identified compounds (AM-694, 3; JWH-122, 5; RCS-4, 4), each product was extracted with methanol under ultrasonication to prepare a sample solution for analysis by liquid chromatography with ultraviolet detection. Each of four commercial products contained some of cannabimimetic indoles 1–5; their contents ranged from 14.8 to 185 mg per pack.






Similar content being viewed by others
References
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Haishima Y, Goda Y (2009) Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem Pharm Bull 57:439–441
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (2009) Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol 27:61–66
Kikura-Hanajiri R, Kawamura M, Maruyama T, Kitajima M, Takayama M, Goda Y (2009) Simultaneous analysis of mitragynine, 7-hydroxymitragynine, and other alkaloids in the psychotropic plant “kratom”(Mitragyna speciosa) by LC–ESI–MS. Forensic Toxicol 27:67–74
Kikuchi H, Uchiyama N, Ogata J, Kikura-Hanajiri R, Goda Y (2010) Chemical constituents and DNA sequence analysis of a psychotropic herbal product. Forensic Toxicol 28:77–83
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2011) Identification and quantitation of two cannabimimetic phenylacetylindoles JWH-251 and JWH-250, and four cannabimimetic naphthoylindoles JWH-081, JWH-015, JWH-200, and JWH-073 as designer drugs in illegal products. Forensic Toxicol 29:25–37
Nakajima J, Takahashi M, Seto T, Suzuki J (2011) Identification and quantitation of cannabimimetic compound JWH-250 as an adulterant in products obtained via the Internet. Forensic Toxicol 29:51–55
Nakajima J, Takahashi M, Seto T, Kanai C, Suzuki J, Yoshida M, Hamano T (2011) Identification and quantitation of two benzoylindoles AM-694 and (4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone, and three cannabimimetic naphthoylindoles JWH-210, JWH-122, and JWH-019 as adulterants in illegal products obtained via the Internet. Forensic Toxicol. doi:10.1007/s11419-011-0108-3
Ministry of Health, Labour and Welfare of Japan (2011) http://search.e-gov.go.jp/servlet/Public?CLASSNAME=PCMMSTDETAIL&id=495100324&Mode=0. Accessed 3 May 2011
Reggio PH (2009) The cannabinoid receptors. Humana Press, New York
Palomäki VA, Lehtonen M, Savinainen JR, Laitinen JT (2007) Visualization of 2-arachidonoylglycerol accumulation and cannabinoid CB1 receptor activity in rat brain cryosections by functional autoradiography. J Neurochem 101:972–981
Martel JC, Ormière AM, Leduc N, Assié MB, Cussac D, Newman-Tancredi A (2007) Native rat hippocampal 5-HT1A receptors show constitutive activity. Mol Pharmacol 71:638–643
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Mato S, Vidal R, Castro E, Diaz A, Pazos A, Valdizán EM (2010) Long-term fluoxetine treatment modulates cannabinoid type 1 receptor-mediated inhibition of adenylyl cyclase in the rat prefrontal cortex through 5-hydroxytryptamine 1A receptor-dependent mechanisms. Mol Pharmacol 77:424–434
Nonaka R, Nagai F, Ogata A, Satoh K (2007) In vitro screening of psychoactive drugs by [35S]GTPγS binding in rat brain membranes. Biol Pharm Bull 30:2328–2333
Makriyannis A, Deng H (2005) Cannabimimetic indole derivatives. United States Patent, US 7,241,799 B2
Willis PG, Katoch-Rouse R, Horti AG (2003) Regioselective F-18 radiolabeling of AM694, a CB1 cannabinoid receptor ligand. J Label Compd Radiopharm 46:799–804
Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson ALS, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR (2005) Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB1 and CB2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB2 receptor agonists. Bioorg Med Chem 13:89–112
Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, Showalter VM, Abood ME, Martin BR (2000) Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60:133–140
Huffman JW, Mabon R, Wu MJ, Lu J, Hart R, Hurst DP, Reggio PH, Wiley JL, Martin BR (2003) 3-Indoyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB1 cannabinoid receptor. Bioorg Med Chem 11:539–549
Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR (1998) Structure-activity relationships of indole and pyrrole-derived cannabinoids. J Pharmacol Exp Ther 285:995–1004
Huffman JW (1999) Cannabimimetic indoles, pyrroles and indenes. Curr Med Chem 6:705–720
EMCDDA (2009) Thematic papers: understanding the “Spice” phenomenon. http://www.emcdda.europa.eu/attachements.cfm/att_80086_EN_Spice%20Thematic%20paper%20%E2%80%94%20final%20version.pdf. Accessed 20 Jan 2011
ACMD (2009) Consideration of the major cannabinoid agonists. http://www.homeoffice.gov.uk/publications/drugs/acmd1/acmd-report-agonists?view=Binary. Accessed January 2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakajima, J., Takahashi, M., Nonaka, R. et al. Identification and quantitation of a benzoylindole (2-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone and a naphthoylindole 1-(5-fluoropentyl-1H-indol-3-yl)-(naphthalene-1-yl)methanone (AM-2201) found in illegal products obtained via the Internet and their cannabimimetic effects evaluated by in vitro [35S]GTPγS binding assays. Forensic Toxicol 29, 132–141 (2011). https://doi.org/10.1007/s11419-011-0114-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-011-0114-5