Abstract
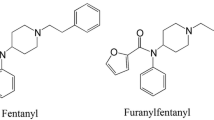
Eleven chemically modified 1-(2-phenethyl)-4-(N-propionylanilino)-piperidine (fentanyl) analogues were synthesized and their analgesic activities were evaluated by the acetic acid writhing method in mice. Their effective dose (ED50) and lethal dose (LD50) values were compared with those of morphine and fentanyl. The synthesized fentanyl analogues were categorized into three groups: a mono-methylated group, a group in which hydrogen in the para-position of the aromatic ring bound to the propionylanilino group was substituted with F, Cl, CH3, or OCH3, and a group in which the propionyl moiety was changed to an acetyl one. 3-Methylfentanyl showed the strongest analgesic activity among these compounds, and the most frequently abused fentanyl derivative, α-methylfentanyl, also showed quite strong activity. The analgesic activities of p-fluorofentanyl and acetylfentanyl were also relatively strong and not negligible. The activities of other analogues were significantly lower than that of fentanyl. The ranges between LD50 and ED50 of these fentanyl analogues were narrower than that of fentanyl. A number of fatal cases in humans caused by the abuse of fentanyl analogues are considered to be due not only to overdosing but also to the narrow ranges between the ED50 and LD50 values.
Similar content being viewed by others
References
Blakeslee S (1985) California addicts use legal, synthetic narcotics. New York Times, March 24
Baum RM (1985) New variety of street drugs poses growing problem. Chem Eng News 63:7–16
Shafer J (1985) Designer drugs. Science 85(March):60–67
Stinson S (1981) Structure of bogus “China White” solved. Chem Eng News 59:71–72
Kram TC, Cooper DA, Allen AC (1981) Behind the identification of China White. Anal Chem 53:1379A–1386A
Cooper D, Jacob M, Allen A (1986) Identification of fentanyl derivatives. J Forensic Sci 31:511–528
Suzuki S, Inoue T, Kashima C (1986) Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and related compounds. I. Spectrometric and chromatographic analyses of 3-methylfentanyl and α-methylfentanyl. Chem Pharm Bull 34:1340–1343
Van Bever WFM, Niemegeers CJE, Janssen PAJ (1974) Synthetic analgesics. Synthesis and pharmacology of the diastereoisomers of N-[3-methyl-1-(2-phenylethyl)-4-piperidyl]-N-phenylpropanamide and N-[3-methyl-1-(1-methyl-2-phenylethyl)-4-piperidyl]-N-phenylpropanamide. J Med Chem 17:1047–1051
Van Bever WFM, Niemegeer CJE, Schellkens KHL, Janssen PAJ (1976) N-4-Substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneim-Forsch 26:1548–1551
Essawi MYH, Portoghese PS (1983) Synthesis and evaluation of 1-and 2-substituted fentanyl analogues for opioid activity. J Med Chem 26:348–352
Borne RF, Fifer EK, Waters IW (1984) Conformationally restrained fentanyl analogs. 2. Synthesis and analgetic evaluation of perhydro-1, 6-naphthyridin-2-ones. J Med Chem 27: 1271–1275
Berger JG, Davidson F, Langford GE (1977) Synthesis of some conformationally restricted analogues of fentanyl. J Med Chem 20:600–602
Grossmann S, Moser U, Mutschler E (1978) Synthese und Pharmakologische Prüfung von Pyridin-Analoga des Fentanyls. Arch Pharm 311:1010–1015
Casy AF, Ogungbamila FO (1982) 3-Allyl analogues of fentanyl. J Pharm Pharmacol 34:210
Ohta H, Suzuki S, Ogasawara K (1999) Studies on fentanyl and related compounds IV. Chromatographic and spectrometric discrimination of fentanyl and its derivatives. J Anal Toxicol 23:280–285
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higashikawa, Y., Suzuki, S. Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues. Forensic Toxicol 26, 1–5 (2008). https://doi.org/10.1007/s11419-007-0039-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-007-0039-1