Abstract
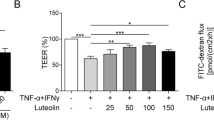
Lentinula edodes mycelia solid culture extract (MSCE) is used as a medical food ingredient and provides beneficial effects to patients with cancer and chronic type C hepatitis. Low molecular weight lignin (LM-lignin), which is an active component of MSCE, exhibits hepatoprotective, antitumor, antiviral, and immunomodulatory effects. In this study, we investigated the effect of LM-lignin/lignosulfonic acid on intestinal barrier function. Lignosulfonic acid enhanced transepithelial membrane electrical resistance in human intestinal Caco-2 cell monolayers. In Caco-2 cells treated with lignosulfonic acid, expression of claudin-2, which forms high conductive cation pores in tight junctions (TJs), was decreased. Lignosulfonic acid also attenuated the barrier dysfunction that is caused by tumor necrosis factor (TNF)-α and interferon (IFN)-γ in Caco-2 cells. TNF-α- and IFN-γ-induced activation of NF-κB, such as translocation of NF-κB p65 into the nucleus and induction of gene expression, was inhibited by lignosulfonic acid treatment. Furthermore, lignosulfonic acid decreased the TNF-α- and IFN-γ-induced increase in interleukin (IL)-1β and IL-6 expression in Caco-2 cells. These results suggest that lignosulfonic acid not only enhances TJ barrier function but also restores TJ barrier integrity impaired by inflammatory cytokines. Therefore, lignosulfonic acid may be beneficial for the treatment of inflammation-induced intestinal barrier dysfunction observed in inflammatory bowel disease.





Similar content being viewed by others
References
Chang R (1996) Functional properties of edible mushrooms. Nutr Rev 54:S91–S93
Chihara G (1992) Recent progress in immunopharmacology and therapeutic effects of polysaccharides. Dev Biol Stand 77:191–197
Akamatsu S, Watanabe A, Tamesada M, Nakamura R, Hayashi S, Kodama D, Kawase M, Yagi K (2004) Hepatoprotective effect of extracts from Lentinus edodes mycelia on dimethylnitrosamine-induced liver injury. Biol Pharm Bull 27:1957–1960
Watanabe A, Kobayashi M, Hayashi S, Kodama D, Isoda K, Kondoh M, Kawase M, Tamesada M, Yagi K (2006) Protection against d-galactosamine-induced acute liver injury by oral administration of extracts from Lentinus edodes mycelia. Biol Pharm Bull 29:1651–1654
Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, Yagi K (2009) Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol Pharm Bull 32:1215–1219
Itoh A, Isoda K, Kondoh M, Kawase M, Watari A, Kobayashi M, Tamesada M, Yagi K (2010) Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury. Biol Pharm Bull 33:983–987
Okuno K, Uno K (2011) Efficacy of orally administered Lentinula edodes mycelia extract for advanced gastrointestinal cancer patients undergoing cancer chemotherapy: a pilot study. Asian Pac J Cancer Prev 12:1671–1674
Shen J, Tanida M, Fujisaki Y, Horii Y, Hashimoto K, Nagai K (2009) Effect of the culture extract of Lentinus edodes mycelia on splenic sympathetic activity and cancer cell proliferation. Auton Neurosci 145:50–54. https://doi.org/10.1016/j.autneu.2008.11.004
Sarkar S, Koga J, Whitley RJ, Chatterjee S (1993) Antiviral effect of the extract of culture medium of Lentinus edodes mycelia on the replication of herpes simplex virus type 1. Antivir Res 20:293–303
Matsuhisa K, Yamane S, Okamoto T, Watari A, Kondoh M, Matsuura Y, Yagi K (2015) Anti-HCV effect of Lentinula edodes mycelia solid culture extracts and low-molecular-weight lignin. Biochem Biophys Res Commun 462:52–57. https://doi.org/10.1016/j.bbrc.2015.04.104
Yamada T, Oinuma T, Niihashi M, Mitsumata M, Fujioka T, Hasegawa K, Nagaoka H, Itakura H (2002) Effects of Lentinus edodes mycelia on dietary-induced atherosclerotic involvement in rabbit aorta. J Atheroscler Thromb 9:149–156
Tanaka K, Ishikawa S, Matsui Y, Tamesada M, Harashima N, Harada M (2011) Oral ingestion of Lentinula edodes mycelia extract inhibits B16 melanoma growth via mitigation of regulatory T cell-mediated immunosuppression. Cancer Sci 102:516–521. https://doi.org/10.1111/j.1349-7006.2010.01841.x
Suzuki H, Iiyama K, Yoshida O, Yamazaki S, Yamamoto N, Toda S (1990) Structural characterization of the immunoactive and antiviral water-solubilized lignin in an extract of the culture medium of Lentinus edodes mycelia (LEM). Agric Biol Chem 54:479–487
Yoshioka Y, Kojima H, Tamura A, Tsuji K, Tamesada M, Yagi K, Murakami N (2012) Low-molecular-weight lignin-rich fraction in the extract of cultured Lentinula edodes mycelia attenuates carbon tetrachloride-induced toxicity in primary cultures of rat hepatocytes. J Nat Med 66:185–191. https://doi.org/10.1007/s11418-011-0580-4
Kaulmann A, Bohn T (2016) Bioactivity of polyphenols: preventive and adjuvant strategies toward reducing inflammatory bowel diseases—promises, perspectives, and pitfalls. Oxid Med Cell Longev 2016:9346470. https://doi.org/10.1155/2016/9346470
Blonski W, Buchner AM, Lichtenstein GR (2011) Inflammatory bowel disease therapy: current state-of-the-art. Curr Opin Gastroenterol 27:346–357. https://doi.org/10.1097/MOG.0b013e328347aef3
Hagan M, Cross RK (2015) Safety of vedolizumab in the treatment of Crohn’s disease and ulcerative colitis. Expert Opin Drug Saf 14:1473–1479. https://doi.org/10.1517/14740338.2015.1063612
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9:799–809. https://doi.org/10.1038/nri2653
Hering NA, Fromm M, Schulzke JD (2012) Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol 590:1035–1044. https://doi.org/10.1113/jphysiol.2011.224568
Hasegawa Y, Kadota Y, Hasegawa C, Kawaminami S (2015) Lignosulfonic acid-induced inhibition of intestinal glucose absorption. J Nutr Sci Vitaminol (Tokyo) 61:449–454. https://doi.org/10.3177/jnsv.61.449
Hasegawa Y, Nakagawa E, Kadota Y, Kawaminami S (2017) Lignosulfonic acid promotes hypertrophy in 3T3-L1 cells without increasing lipid content and increases their 2-deoxyglucose uptake. Asian-Australas J Anim Sci 30:111–118. https://doi.org/10.5713/ajas.16.0253
Qiu M, Wang Q, Chu Y, Yuan Z, Song H, Chen Z, Wu Z (2012) Lignosulfonic acid exhibits broadly anti-HIV-1 activity—potential as a microbicide candidate for the prevention of HIV-1 sexual transmission. PLoS One 7:e35906. https://doi.org/10.1371/journal.pone.0035906
Gordts SC, Ferir G, D’Huys T, Petrova MI, Lebeer S, Snoeck R, Andrei G, Schols D (2015) The low-cost compound lignosulfonic acid (LA) exhibits broad-spectrum anti-HIV and anti-HSV activity and has potential for microbicidal applications. PLoS One 10:e0131219. https://doi.org/10.1371/journal.pone.0131219
Watari A, Hasegawa M, Yagi K, Kondoh M (2016) Checkpoint kinase 1 activation enhances intestinal epithelial barrier function via regulation of claudin-5 expression. PLoS One 11:e0145631. https://doi.org/10.1371/journal.pone.0145631
Watari A, Sakamoto Y, Hisaie K, Iwamoto K, Fueta M, Yagi K, Kondoh M (2017) Rebeccamycin attenuates TNF-alpha-induced intestinal epithelial barrier dysfunction by inhibiting myosin light chain kinase production. Cell Physiol Biochem 41:1924–1934. https://doi.org/10.1159/000472367
Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, Ciclitira PJ, Al-Hassi HO (2016) Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol 22:3117–3126. https://doi.org/10.3748/wjg.v22.i11.3117
Takehara M, Nishimura T, Mima S, Hoshino T, Mizushima T (2009) Effect of claudin expression on paracellular permeability, migration and invasion of colonic cancer cells. Biol Pharm Bull 32:825–831
Lee SH (2015) Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13:11–18. https://doi.org/10.5217/ir.2015.13.1.11
Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM (2004) TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 286:G367–G376. https://doi.org/10.1152/ajpgi.00173.2003
He F, Peng J, Deng XL, Yang LF, Camara AD, Omran A, Wang GL, Wu LW, Zhang CL, Yin F (2012) Mechanisms of tumor necrosis factor-alpha-induced leaks in intestine epithelial barrier. Cytokine 59:264–272. https://doi.org/10.1016/j.cyto.2012.04.008
Chen S, Zhu J, Chen G, Zuo S, Zhang J, Chen Z, Wang X, Li J, Liu Y, Wang P (2015) 1,25-Dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-alpha induced injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling pathway. Biochem Biophys Res Commun 460:873–878. https://doi.org/10.1016/j.bbrc.2015.03.125
Chen SW, Zhu J, Zuo S, Zhang JL, Chen ZY, Chen GW, Wang X, Pan YS, Liu YC, Wang PY (2015) Protective effect of hydrogen sulfide on TNF-alpha and IFN-gamma-induced injury of intestinal epithelial barrier function in Caco-2 monolayers. Inflamm Res 64:789–797. https://doi.org/10.1007/s00011-015-0862-5
Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR (2006) Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 119:2095–2106. https://doi.org/10.1242/jcs.02915
Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN (2012) Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24:503–512. https://doi.org/10.1111/j.1365-2982.2012.01921.x
Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2007) Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56:61–72. https://doi.org/10.1136/gut.2006.094375
Oshima T, Miwa H, Joh T (2008) Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol 23(Suppl 2):S146–S150. https://doi.org/10.1111/j.1440-1746.2008.05405.x
Furuse M, Furuse K, Sasaki H, Tsukita S (2001) Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153:263–272
Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD (2010) TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci 123:4145–4155. https://doi.org/10.1242/jcs.070896
Al-Sadi R, Guo S, Ye D, Rawat M, Ma TY (2016) TNF-alpha modulation of intestinal tight junction permeability is mediated by NIK/IKK-alpha axis activation of the canonical NF-kappaB pathway. Am J Pathol 186:1151–1165. https://doi.org/10.1016/j.ajpath.2015.12.016
Al-Sadi R, Guo S, Ye D, Ma TY (2013) TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol 183:1871–1884. https://doi.org/10.1016/j.ajpath.2013.09.001
Al-Sadi RM, Ma TY (2007) IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 178:4641–4649
Suzuki T, Yoshinaga N, Tanabe S (2011) Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 286:31263–31271. https://doi.org/10.1074/jbc.M111.238147
Ina K, Kusugami K, Yamaguchi T, Imada A, Hosokawa T, Ohsuga M, Shinoda M, Ando T, Ito K, Yokoyama Y (1997) Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol 92:1342–1346
Acknowledgements
We thank Ms. Shizuka Yamagishi for technical assistance. This work was supported by JSPS KAKENHI Grant Nos. 26860078 and 16K08370; by the Suzuken Memorial Foundation; by the Mishima Kaiun Memorial Foundation; by the Yokoyama Rinsho Yakuri Foundation; and by the Basic Science and Platform Technology Program for innovative Biological Medicine from AMED.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuhisa, K., Watari, A., Iwamoto, K. et al. Lignosulfonic acid attenuates NF-κB activation and intestinal epithelial barrier dysfunction induced by TNF-α/IFN-γ in Caco-2 cells. J Nat Med 72, 448–455 (2018). https://doi.org/10.1007/s11418-017-1167-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-017-1167-5