Abstract
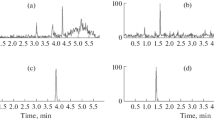
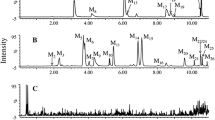
Buyang Huanwu decoction (BYHWD), a famous traditional Chinese medicine prescription for the treatment of cerebrovascular diseases, is composed of seven commonly used Chinese herbs—Astragali Radix, Angelicae Sinensis Radix, Paeoniae Radix Rubra, Chuanxiong Rhizoma, Carthami Flos, Persicae Semen and Pheretima. To determine the main absorptive constituents and the metabolites of BYHWD in vivo, urine samples from Wuzhishan (WZS) miniature pigs orally administered with BYHWD (13.6 g crude drugs/kg) were collected to investigate the characteristic compounds. By comparing the high-performance liquid chromatography of a drug-containing urine sample with that of a drug-free sample, 17 characteristic compounds were isolated from the methanol extract of a drug-containing urine sample by column chromatography. Their structures, including 11 isoflavanoids, 2 pterocarpanoids and 4 isoflavonoids, were identified by spectroscopic means. Of the 17 compounds, 8 (1–8) were new compounds with the following structures: 3S-7,3′,4′-trihydroxyisoflavan-3′-O-β-d-glucuronide (1), 3S-7,3′,4′-trihydroxyisoflavan-4′-O-β-d-glucuronide (2), 3S-7,2′,4′-trihydroxyisoflavan-2′-O-β-d-glucuronide (3), 3R-7,2′-dihydroxy-3′,4′-dimethoxyisoflavan-2′-O-β-d-glucuronide (4), 3R-7,2′-dihydroxy-3′,4′-dimethoxyisoflavan-2′-O-β-d-glucuronide-6″-methyl ester (5), 3R-7,2′-dihydroxy-3′,4′-dimethoxyisoflavan-7-O-β-d-glucuronide-6″-methyl ester (6), 3R-7,2′,3′-trihydroxy-4′-methoxyisoflavan-3′-O-β-d-glucuronide-6″-methyl ester (7), and 3S-7,4′,5′-trihydroxy-2′,3′-dimethoxyisoflavan-5′-O-β-d-glucuronide (8). Based on the possible relationship and metabolic pathways of the 17 compounds in vivo, 3R-7,2′-dihydroxy-3′,4′-dimethoxyisoflavan (isomucronulatol, 11), 6aR,11aR-3-hydroxy-9,10-dimethoxypterocarpan (methylnissolin, astrapterocarpan, 13), 7,3′-dihydroxy-4′-methoxyisoflavone (calycosin, 16) and 7-hydroxy-4′-methoxyisoflavone (formononetin, 17) were thought to be the most important absorptive original isoflavonoid constituents of BYHWD in vivo, which underwent reactions of glucuronidation, hydroxylation, demethylation and reduction. The other 13 compounds were deduced to be their metabolites.





Similar content being viewed by others
References
Cai G-X, Liu B-Y (2010) Effect of ultra-micronized Buyang Huanwu decoction on neurological function, quality of life, and serum vascular endothelial growth factor in patients convalescent from cerebral infarction. Chin Crit Care Med 22:591–594
Wu Y-S, Jiang L-P (1998) Clinical study on buyanghuanwu decoction to the metabolic imbalance of endothelin and calcitonin gene related peptide in patients with early cerebral infarction. Chin J Integr Tradit West Med 18:396–398
Zhang H, Liang M-J, Ma Z-X, Ye S-L (1995) Clinical study on effects of buyanghuanwu decoction on coronary heart disease. Chin J Integr Tradit West Med 15:213–215
Zha L-L, Shen Z-Y, Zhang P (1994) Clinical and experimental research of buyanghuanwu tang granule in treatment of ischemic apoplexy. Chin J Integr Tradit West Med 14:74–76, 67
Shen Q, Zheng Q-J (1993) Effect of photosensitized oxidation auto-hemotherapy with buyanghuanwu tang on sequela of apoplexy. Chin J Integr Tradit West Med 13:402–404, 387
Chu C, Qi L-W, Liu E-H, Li B, Gao W, Li P (2010) Radix Astragali (Astragalus): latest advancements and trends in chemistry, analysis, pharmacology and pharmacokinetics. Curr Org Chem 14:1792–1807
Ran X, Ma L, Peng C, Zhang H, Qin L-P (2011) Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharm Biol 49:1180–1189
Liu X, Li W, Cai B (2010) Advances in research of chemical constituents and the pharmacological activities on cardio- and cerebro-vascular systems of Angelicae Sinensis Radix. J Nanjing TCM Univ 26:155–157
Ruan J-L, Zhao Z-X, Zeng Q-Z, Qian Z-M (2003) Recent advances in study of components and pharmacological roles of Radix Paeoniae Rubra. Chin Pharmacol Bull 19:965–970
Sarengaowa, Chen H-M, Quan S (2009) Summary of the research on chemical compositions and pharmacological activities of Mongolian drug Carthamus tinctorius L. J Inner Mongolia Univ Natl 24:333–336
Wang R-F, Fan L-G, Gao W-Y, Zhang J-Y (2010) Advances in research of chemical constituents and pharmacological activities of Persicae Semen. Drug Clin 25:426–429
Yang D, Cai S, Liu H, Guo X, Li C, Shang M, Wang X, Zhao Y (2006) On-line identification of the constituents of Buyang Huanwu decoction in pig serum using combined HPLC–DAD–MS techniques. J Chromatogr B 831:288–302
Muthyala R-S, Ju Y-H, Sheng S, Williams L-D, Doerge D-R, Katzenellenbogen B-S, Helferich W-G, Katzenellenbogen J-A (2004) Equol, a natural estrogenic metabolite from soy isoflavones convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem 12:1559–1567
Piccinelli A-L, Campo F-M, Cuesta-Rubio O, Marquez H-I, De Simone F, Rastrelli L (2005) Isoflavonoids isolated from Cuban Propolis. J Agric Food Chem 53:9010–9016
Luk K-C, Stern L, Weigele M, O’Brien R-A, Spirt N (1983) Isolation and identification of “diazepam-like” compounds from bovine urine. J Nat Prod 46:852–861
Chang Y-C, Nair M-G (1995) Metabolism of daidzein and genistein by intestinal bacteria. J Nat Prod 58:1892–1896
Liang G, Zhang T, Wang J, Chen B, Cheng K, Hu C (2005) X-ray single-crystal analysis of (−)-(S)-equol isolated from rat’s feces. Chem Biodivers 2:959–963
Setchell K-D-R, Brown N-M, Lydeking-Olsen E (2002) The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr 132:3577–3584
He Z-Q, Findlay J-A (1991) Constituents of Astragalus membranaceus. J Nat Prod 54:810–815
Subarnas A, Oshima Y, Hikino H (1991) Isoflavans and a pterocarpan from Astragalus mongholicus. Phytochemistry 30:2777–2780
Zhao M, Duan J-A, Huang W-Z, Zhou R-H, Che Z-T (2002) Isoflavans and isoflavone from Astragalus hoantchy. J Chin Pharm Univ 33:274–276
Robeson D-J, Ingham J-L (1979) New pterocarpan phytoalexins from Lathyrus nissolia. Phytochemistry 18:1715–1717
Spencer G-F, Jones B-E, Plattner R-D, Barnekow D-E, Brinen L-S, Clardy J (1991) A pterocarpan and two isoflavans from alfalfa. Phytochemistry 30:4147–4149
Du X, Bai Y, Liang H, Wang Z, Zhao Y, Zhang Q, Huang L (2006) Solvent effect in 1H NMR spectra of 3′-hydroxy-4′-methoxy isoflavonoids from Astragalus membranaceus var. mongholicus. Magn Reson Chem 44:708–712
Herath H-M-T-B, Dassanayake R-S, Priyadarshani A-M-A, De Silva S, Wannigama G-P, Jamie J (1998) Isoflavonoids and a pterocarpan from Gliricidia sepium. Phytochemistry 47:117–119
Kamnaing P, Fanso Free S-N-Y, Nkengfack A-E, Folefoc G, Fomum Z-T (1999) An isoflavan-quinone and a flavonol from Millettia laurentii. Phytochemistry 51:829–832
Salem M-M, Werbovetz K-A (2006) Isoflavonoids and other compounds from Psorothamnus arborescens with antiprotozoal activities. J Nat Prod 69:43–49
Kobayashi M, Noguchi H, Sankawa U (1985) Formation of chalcones and isoflavones by callus culture of Glycyrrhiza uralensis with different production patterns. Chem Pharm Bull 33:3811–3816
Heinonen S-M, Hoikkala A, Wahala K, Adlercreutz H (2003) Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects. Identification of new metabolites having an intact isoflavonoid skeleton. J Steroid Biochem Mol Biol 87:285–299
Heinonen S-M, Wahala K, Adlercreutz H (2004) Identification of urinary metabolites of the red clover isoflavones formononetin and biochanin A in human subjects. J Agric Food Chem 52:6802–6809
Kulling S-E, Lehmann L, Metzler M (2002) Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J Chromatogr B 777:211–218
Rufer C-E, Glatt H, Kulling S-E (2006) Structural elucidation of hydroxylated metabolites of the isoflavan equol by gas chromatography–mass spectrometry and high-performance liquid chromatography–mass spectrometry. Drug Metab Dispos 34:51–60
Ohkawara S, Okuma Y, Uehara T, Yamagishi T, Nomura Y (2005) Astrapterocarpan isolated from Astragalus membranaceus inhibits proliferation of vascular smooth muscle cells. Eur J Pharmacol 525:41–47
Lu J-F, Li C-X, Muteliefu G, Li T-F, Tu P-F, Yin J-J, Cai S-Q (2002) Effects of BYHW decoction and its effective constituents on the fluidity of the cell membrane in a stroke-modeled rat brain. J Chin Pharm Sci 11:132–136
Acknowledgments
This work was supported by National Natural Science Foundation of P. R. China (Grant No. 30371721) and State Key Program of National Natural Science of the People’s Republic of China (Grant No. 30830120).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, DH., Ren, XL., Xu, F. et al. Absorptive constituents and their metabolites in drug-containing urine samples from Wuzhishan miniature pigs orally administered with Buyang Huanwu decoction. J Nat Med 68, 11–21 (2014). https://doi.org/10.1007/s11418-013-0756-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-013-0756-1