Abstract
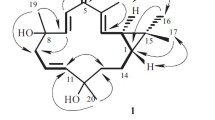
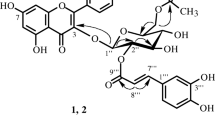
A chemical investigation of the chloroform extract of the roots of Uvaria welwitschii (Annonaceae), an African traditional medicine taken for stomach ache, led to the isolation of eight new compounds, named welwitschins A–H (1–8), together with five known compounds (9–13). The structural elucidation by spectroscopic studies of the compounds isolated is described. All new compounds were flavonoids having a 2-hydroxy-3,4,6-trimethoxyphenyl moiety in the A-ring, and unsubstituted phenyl in the B-ring. Four of them (1–4) were monomeric flavonoids while the others (5–8) were dimeric flavonoids. The cytotoxicity of the isolated compounds against human promyelocytic leukemia HL-60 cells was investigated.







Similar content being viewed by others
References
Verdcourt B (1971) Flora of Tropical East Africa, Annonaceae. The East African Community, Nairobi, Kenya, pp 24–25
Ichimaru M, Nakatani N, Takahashi T, Nishiyama Y, Moriyasu M, Kato A, Mathenge SG, Juma FD, Nganga JN (2004) Cytotoxic C-benzylated dihydrochalcones from Uvaria acuminata. Chem Pharm Bull 52:138–141
Ichimaru M, Nakatani N, Moriyasu M, Nishiyama Y, Kato A, Mathenge SG, Juma FD, ChaloMutiso PB (2010) Hydroxyespintanol and schefflerichalcone, two new compounds from Uvaria scheffleri. J Nat Med 64:75–79
Iinuma M, Matoba Y, Tanaka T, Mizuno M (1986) Flavonoids synthesis. I. Synthesis and spectroscopic properties of flavones with two hydroxy and five methoxy groups at C-2′, 3′, 4′, 5, 6, 6′, 7 and C-2′, 3, 4′, 5, 5′, 6, 7. Chem Pharm Bull 34:1656–1662
Colegate SM, Din LB, Ghisalberti EL, Latiff A (1992) Tepanone, a retrochalcone from Ellipeia cuneifolia. Phytochemistry 31:2123–2126
Lien TP, Porzel A, Schmidt J, Van SY, Adam G (2000) Chalconoids from Fissistigma bracteolatum. Phytochemistry 53:991–995
Adinarayana D, Gunasekar D (1976) A new flavone from Actinodaphne madraspatana. Indian J Chem 18B:552
Nkunya MHH, Waibel R, Achenbach H (1993) Antimalarials and other constituents of plants of the genus Uvaria. Part. 9. Three flavonoids from the stem bark of the antimalarial Uvaria dependens. Phytochemistry 34:853–856
Paul EG, Wang PSC (1979) Reaction of 2,3,4,6-tetramethoxybenzaldehyde with aluminum chloride. Selective cleavage at position 2 and selective ether exchange at position 3. J Org Chem 44:2307–2308
Royer R, Rene L, Cavire R, Lemoine J (1977) Research on nitro-derivatives of biological interest. XIII. Synthesis and protozoacidal and antibacterial activities of polymethoxylated derivatives of 2-nitrobenzofuran. Euro J Med Chem 12:455–458
Mohamed KM, Hassannean HA, Ohtani K, Kasai R, Yamasaki K (2000) Chalcanel glucosides from seeds of Trifolium alexandrinum. Phytochemistry 53:401–404
Nishide K, Shigeta Y, Obata K, Node M (1996) Asymmetric 1, 7-hydride shift The highly asymmetric reduction of α, β-unsaturated ketones to secondary alcohols via a novel tandem Michael addition/Meerwein-Ponndorf-Verley reduction. J Am Chem Soc 118:13103–13104
Skarzewski J, Siedlecka R, Wojaczynska E, Zielinska-Btajet M (2002) A new and efficient route to homochiral γ-hydroxysulfoxides and γ-hydroxysulfones. Tetrahedron Asymmetr 13:2105–2111
Node M, Nishide K, Shigeta Y, Shiraki H, Obata K (2000) Novel tandem Michael Addition/Meerwein-Ponndorf-Verley reduction asymmetric reduction of acyclic α, β-unsaturated ketones using a chiral mercapto alcohol. J Am Chem Soc 122:1927–1936
Holt DA, Luengo JI, Yamashita DS, Oh H, Konialian AL, Yen HK, Rozamus LW, Brandt M, Bossard MJ (1993) Design, synthesis, and kinetic evaluation of high-affinity FKBP ligands and the X-ray crystal structures of their complexes with FKBP12, A. J Am Chem Soc 115:9925–9938
Belzecki C, Panfil I (1979) Cycloaddition of chiral nitrones asymmetric synthesis of isoxazolidines. J Org Chem 44:1212–1218
Ohtani I, Kusumi T, Kashman Y, Kakisawa H (1991) High-field FT NMR application of Mosher’s method The absolute configurations of marine terpenoids. J Am Chem Soc 113:4092–4096
Kusumi T (1993) Determination of the absolute configuration of organic compounds by means of NMR spectroscopy. Modified Mosher’s method. Yuki Gosei Kyokaishi 51:462–470 (in Japanese)
Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K (1997) A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 44:1299–1305
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moriyasu, M., Nakatani, N., Ichimaru, M. et al. Chemical studies on the roots of Uvaria welwitschii . J Nat Med 65, 313–321 (2011). https://doi.org/10.1007/s11418-010-0498-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-010-0498-2