Abstract
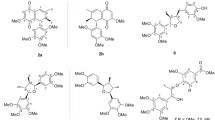
A novel α-tetralonyl derivative, juglanone, was isolated from the fresh unripe fruits of Juglans mandshurica. Its structure was determined by spectroscopic analyses and from chemical evidence. Juglanone exhibited significant antioxidant activity in assays for 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity and superoxide dismutase-like activity with IC50 values of 10.1 and 9.0 μM, respectively. It also showed moderate cytotoxic activity against HL-60 human myeloid leukemia with an IC50 value of 19.7 μM.



Similar content being viewed by others
References
Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49:835–842
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Lovell MA, Markesbery WR (2007) Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res 35:7497–7504
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Perez-Matute P, Zulet MA, Martinez JA (2009) Reactive species and diabetes: counteracting oxidative stress to improve health. Curr Opin Pharmacol 9:771–779
Mates JM, Segura JA, Alonso FJ, Marquez J (2009) Natural antioxidants: therapeutic prospects for cancer and neurological diseases. Mini Rev Med Chem 9:1202–1214
State Administration of Traditional Chinese Medicine “Zhong Hua Ben Cao” Editorial Committee (1999) Zhong Hua Ben Cao, vol 2. Shanghai Scientific Technological Publishers, Shanghai, pp 373–375
Li ZB, Wang JY, Jiang B, Zhang XL, An LJ, Bao YM (2007) Benzobijuglone, a novel cytotoxic compound from Juglans mandshurica, induced apoptosis in HeLa cervical cancer cells. Phytomedicine 14(12):846–852
Han L, Li W, Narimatsu S, Liu L, Fu H, Okuda H, Koike K (2007) Inhibitory effects of compounds isolated from fruit of Juglans mandshurica on pancreatic lipase. J Nat Med 61(2):184–186
Machida K, Matsuoka E, Kasahara T, Kikuchi M (2005) Studies on the constituents of Juglans species. I. Structural determination of (4S)- and (4R)-4-hydroxy-α-tetralone derivatives from the fruit of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. Chem Pharm Bull 53(8):934–937
Li L, Satou T, Koike K, Li W, Nikaido T (2004) Studies on the cytotoxicity of compounds from fruits of Juglans mandshurica. Nat Med 58(5):226–229
Liu L, Li W, Koike K, Nikaido T (2004) Two new naphthalenyl glucosides and a new phenylbutyric acid glucoside from the fruit of Juglans mandshurica. Heterocycles 63(6):1429–1436
Li L, Li W, Koike K, Zhang S, Nikaido T (2004) New α-tetralonyl glucosides from the fruit of Juglans mandshurica. Chem Pharm Bull 52(5):566–569
Lee EB, Hyun JE, Li DW, Jeong CS, Seung SW, Cho SI, Jhon GJ, Lee EH (2001) Gastroprotective activity of the unripe fruit extract of Juglans mandshurica in rats. Nat Prod Sci 7(3):87–89
Du X, Li Y, Ni Y, Gao K, Zhou D, Yang G (2000) Study on analgesic effect of young fruit of Juglans mandshurica Maxim and its clinical use. Zhongguo Zhongyao Zazhi 25(1):7–10
Müller W, Leistner E (1976) 1,4-Naphthoquinone, an intermediate in juglone (5-hydroxy-1,4-naphthoquinone) biosynthesis. Phytochemistry 15:407–410
Inoue K, Ueda S, Shiobara Y, Inouye H (1980) Stereochemistry of prenylation and subsequent decarboxylation in the biosynthesis of prenylnaphthoquinone congeners in callus cultures of Catalpa ovate. Tetrahedron Lett 21:621–622
Park JS, Park HY, Kim DH, Kim DH, Kim HK (2008) ortho-Dihydroxyisoflavone derivatives from aged Doenjang (Korean fermented soypaste) and its radical scavenging activity. Bioorg Med Chem Lett 18:5006–5009
Chang X, Li W, Jia Z, Satou T, Fushiya S, Koike K (2007) Biologically active triterpenoid saponins from Ardisia japonica. J Nat Prod 70:179–187
Acknowledgments
We thank Dr. Yoshio Hano for helpful discussions concerning the manuscript. L. L. is grateful to Youth Fund of Education Department of Heilongjiang Province of China (11531279) and Youth Fund of Science and Technology Department of Heilongjiang Province of China (QC07C65).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Li, W., Sasaki, T. et al. Juglanone, a novel α-tetralonyl derivative with potent antioxidant activity from Juglans mandshurica . J Nat Med 64, 496–499 (2010). https://doi.org/10.1007/s11418-010-0435-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-010-0435-4