Abstract
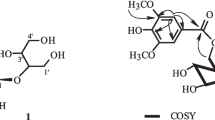
Two chalcone derivatives, 2′-hydroxychalcone (1) and desmethylinfectocaryone (2), together with five known phenolic compounds infectocaryone (3), cryptocaryone (4), kurzichalcolactone A (5), pinocembrin (6) and trans-N-feruloyltyramine (7), were isolated from the methanol extract of the wood of Cryptocarya konishii. The structures of the new compounds were determined based on the analysis of spectroscopic data, including UV, IR, 1D and 2D NMR, and mass spectra. Evaluation of the cytotoxic and tyrosine kinase inhibitory activities of compounds 1–7 showed that compounds 2–4 strongly inhibited the growth of murine leukemia P-388 cells, whereas compound 4 significantly inhibited the enzyme.

Similar content being viewed by others
References
Cronquist A (1981) An integrated system of classification of flowering plants. Columbia University Press, New York, pp 74–78
Awang K, Hadi AHA, Saidi N, Mukhtar MR, Morita H, Litaudon M (2008) New phenantrene alkaloids from Cryptocarya crassinervia. Fitoterapia 79:308–310
Toribio A, Bonfils A, Delannay E, Prost E, Harakat D, Henon E, Richard B, Litaudon M, Nuzillard J-M, Renault J-H (2006) Novel seco-dibenzopyrrocoline alkaloid from Cryptocarya oubatchensis. Org Lett 8:3825–3828
Lin F-W, Wang J-J, Wu T-S (2002) New pavine N-oxide alkaloids from the stem bark of Cryptocarya chinensis Hemsl. Chem Pharm Bull 50:157–159
Dumontet V, Van Hung N, Adeline M-T, Riche C, Chiaroni A, Sevenet T, Gueritte F (2004) Cytotoxic flavonoids and α-pyrones from Cryptocarya obovata. J Nat Prod 67:858–862
Chan Y-Y, Wu C-H, Wu S-J, Wu T-S (2002) The constituents and synthesis of cryptamygin-A from the stem bark of Cryptocarya amygdalina. J Chin Chem Soc 49:263–268
Schmeda-Hirschmann G, Astudillo L, Bastida J, Codina C, De Arias AR, Ferreira ME, Inchaustti A, Yaluff G (2001) Cryptofolione derivatives from Cryptocarya alba fruits. J Pharm Pharmacol 53:563–567
Juliawaty LD, Kitajima M, Takayama H, Achmad SA, Aimi N (2000) A 6-substituted-5,6-dihydro-2-pyrone from Cryptocarya strictifolia. Phytochemistry 54:989–993
Usman H, Hakim EH, Harlim T, Jalaluddin MN, Syah YM, Achmad SA, Takayama H (2006) Cytotoxic chalcones and flavanones from the tree bark of Cryptocarya costata. Z Naturforsch 61c:184–188
Dumontet V, Gaspard C, Van Hung N, Fahy J, Tchertanov L, Sevenet T, Gueritte F (2001) New cytotoxic flavonoids from Cryptocarya infectoria. Tetrahedron 57:6189–6196
Lu S-T (1966) Alkaloids of Formosan lauraceous plants. IX. Alkaloids of Cryptocarya chinensis and C. konishii. Yakugaku Zasshi 86:296–299
Lu S-T (1967) Alkaloids of Formosan lauraceous plants. XII. Alkaloids of Cryptocarya konishii and Machilus acuminatissimus. Yakugaku Zasshi 87:1278–1281
Lee SS, Lin YJ, Chen CK, Liu KCS, Chen CH (1993) Quaternary alkaloids from Litsea cubeba and Cryptocarya konishii. J Nat Prod 56:1971–1976
Govindachari TR, Parthasarathy PC, Desai HK, Shanbhag MN (1973) Structure of cryptocaryone. Constituent of Cryptocarya bourdilloni. Tetrahedron 29:3091–3094
Wagner H, Chari VM, Sonnenbichler J (1976) 13C-NMR-spektren naturlich vorkommender flavonoide. Tetrahedron Lett 17:1799–1802
Fu X, Sevenet T, Hamid A, Hadi A, Remy F, Pais M (1993) Kurzilactone from Cryptocarya kurzii. Phytochemistry 33:1272–1274
Yoshihara T, Yamaguchi K, Takamatsu S, Sakamura S (1981) A new lignan amide, grossamide, from bell pepper (Capsicum annuum var. grossum). Agric Biol Chem 45:2593–2598
Sahidin HEH, Juliawaty LD, Syah YM, Din LB, Ghisalberti EL, Latip J, Said IM, Achmad SA (2005) Cytotoxic properties of oligostilbenoids from the tree bark of Hopea dryobalanoides. Z Naturforsch 60c:723–727
Acknowledgments
This study was supported by a JSPS (The Japan Society for the Promotion of Science) grant to one of us (FK) through Meiji Pharmaceutical University Asia/Africa Centre for Drug Discovery Program, Japan. Financial assistance from a Doctoral Research Grant, Higher Education Bureau, National Education Department of Republic Indonesia, is also gratefully acknowledged. We thank Cibodas Botanical Garden, West Java, Indonesia, for supplying and identifying the sample.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurniadewi, F., Juliawaty, L.D., Syah, Y.M. et al. Phenolic compounds from Cryptocarya konishii: their cytotoxic and tyrosine kinase inhibitory properties. J Nat Med 64, 121–125 (2010). https://doi.org/10.1007/s11418-009-0368-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-009-0368-y