Abstract
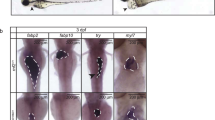
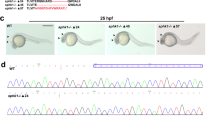
Zebrafish tbx5 expresses in the heart, pectoral fins and eyes of zebrafish during embryonic development. In zebrafish, injection of tbx5 morpholino antisense RNA caused changes of heart conformation, defect of heart looping, pericardium effusion, dropsy of ventral position and decreased heart rate. We suggested that cardiac myogenesis genes might be responsible for this phenomenon. Morpholino antisense RNA which against the initiation site of tbx5 gene was designed in order to knockdown the expression of tbx5, and the results were analyzed by whole-mount in situ hybridization and quantitative real-time PCR. Expression of cardiac myogenesis genes amhc, vmhc and cmlc2 were expressed constantly at the early embryonic development and reached its highest rate right before cardiac looping initiated. These cardiac myogenesis genes showed insufficient expressions within different heart defect embryos. Moreover, vmhc showed ectopic expression in addition to heart looping defect in heart defective embryos at 36 hpf. Our data suggests that the heart failure caused by the knockdown of tbx5 gene might result from the down-regulation of cardiac myogenesis genes.






Similar content being viewed by others
References
Smith J (1999) T-box genes: what they do and how they do it. Trends Genet 15:154–158
Tada M, Smith JC (2001) T-targets: clues to understanding the functions of T-box proteins. Dev Growth Differ 43:1–11
Murray JC (2001) Time for T. Nat Genet 29:107–109
Reamon-Buettner SM, Borlak J (2004) TBX5 mutations in non-Holt-Oram syndrome (HOS) malformed hearts. Hum Mutat 24: 104
Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J et al (1997) Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet 15:30–35
Horb ME, Thomsen GH (1996) Tbx5 is essential for heart development. Development 126:1739–1751
Garrity DM, Childs S, Fishman MC (2002) The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development 129:4635–4645
Holt M, Oram S (1960) Familial heart disease with skeletal malformations. Br Heart J 22:236–242
Yutzey KE, Benson DW (2003) TBX5: a developmental key that fits many locks. J Mol Cell Cardiol 35:1175–1177
Fan C, Duhagon MA, Oberti C, Chen S, Hiroi Y, Komuro I et al (2003) Novel TBX5 mutations and molecular mechanism for Holt-Oram syndrome. J Med Genet 40:e29
Fan C, Liu M, Wang Q (2003) Functional analysis of TBX5 missense mutations associated with Holt-Oram syndrome. J Biol Chem 278:8780–8785
Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S et al (2001) A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106:709–721
Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R et al (2001) Tbx5 associates with Nkx2–5 and synergistically promotes cardiomyocyte differentiation. Nat Genet 28:276–280
Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA et al (2003) GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 424:443–447
Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP et al (1998) Congenital heart disease caused by mutations in the transcription factor NKX2–5. Science 281:108–111
Cleaver OB, Patterson KD, Krieg PA (1996) Overexpression of the tinman-related genes XNkx-2.5 and XNkx-2.3 in Xenopus embryos results in myocardial hyperplasia. Development 122:3549–3556
Hatcher CJ, Diman NY, Kim MS, Pennisi D, Song Y, Goldstein MM et al (2004) A role for Tbx5 in proepicardial cell migration during cardiogenesis. Physiol Genomics 18:129–140
Schulte-Merker S, Ho RK, Herrmann BG, Nüsslein-Volhard C (1992) The protein production of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116:1021–1032
Oxtoby E, Jowett T (1993) Cloning of the zebrafish krox–20 gene (krx–20) and its expression during hindbrain development. Nucleic Acids Res 21:1087–1095
Yelon D, Horne SA, Stainier DY (1999) Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol 214:23–37
Yutzey KE, Rhee JT, Bader D (1994) Expression of the atrial-specific myosin heavy chain AMHC1 and the establishment of anteroposterior polarity in the developing chicken heart. Development 120:871–883
Keegan BR, Meyer D, Yelon D (2004) Organization of cardiac chamber progenitors in the zebrafish blastula. Development 131:3081–3091
Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ (2003) Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn 228:30–40
Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D (2003) Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development 130:6121–6129
Auman HJ, Coleman H, Riley HE, Olale F, Tsai HJ, Yelon D (2007) Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol 5:e53
Rottbauer W, Wessels G, Dahme T, Just S, Trano N, Hassel D et al (2006) Cardiac myosin light chain–2: a novel essential component of thick-myofilament assembly and contractility of the heart. Circ Res 99:323–331
Brown DD, Martz SN, Binder O, Goetz SC, Price BM, Smith JC et al (2005) Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development 132:553–563
Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN (1997) Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet 16:154–160
Latacha KS, Remond MC, Ramasubramanian A, Chen AY, Elson EL, Taber LA (2005) Role of actin polymerization in bending of the early heart tube. Dev Dyn 233:1272–1286
Linask KK, Vanauker M (2007) A role for the cytoskeleton in heart looping. ScientificWorldJournal 7:280–298
Anversa P, Nadal-Ginard B (2002) Cardiac chimerism: methods matter. Circulation 106:e129–e131
Murry CE, Kay MA, Bartosek T, Hauschka SD, Schwartz SM (1996) Muscle differentiation during repair of myocardial necrosis in rats via gene transfer with MyoD. J Clin Invest 98:2209–2217
Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M et al (2006) Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99:15–24
Manner J (2000) Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec 259:248–262
Lin Q, Schwarz J, Bucana C, Olson EN (1997) Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276:1404–1407
Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L et al (1995) Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev 9:1654–1666
Tsuda T, Philp N, Zile MH, Linask KK (1996) Left-right asymmetric localization of flectin in the extracellular matrix during heart looping. Dev Biol 173:39–50
Wohlgemuth SL, Crawford BD, Pilgrim DB (2007) The myosin co-chaperone UNC–45 is required for skeletal and cardiac muscle function in zebrafish. Dev Biol 303:483–492
Cheng L, Guo XF, Yang XY, Chong M, Cheng J, Li G et al (2006) Delta-sarcoglycan is necessary for early heart and muscle development in zebrafish. Biochem Biophys Res Commun 344:1290–1299
Manasek FJ, Monroe RG (1972) Early cardiac morphogenesis is independent of function. Dev Biol 27:584–588
Nakamura A, Manasek FJ (1981) An experimental study of the relation of cardiac jelly to the shape of the early chick embryonic heart. J Embryol Exp Morphol 65:235–256
Acknowledgements
We thank Professor C.H. Hu and C.Y. Ko from Institute of Bioscience and Biotechnology, National Taiwan Ocean University for providing GFP construct and helpful suggestion for the construction of the tbx5-GFP construct.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jen Her Lu and Jenn Kan Lu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, J.H., Lu, J.K., Choo, S.L. et al. Cascade effect of cardiac myogenesis gene expression during cardiac looping in tbx5 knockdown zebrafish embryos. J Biomed Sci 15, 779–787 (2008). https://doi.org/10.1007/s11373-008-9268-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11373-008-9268-5