Abstract
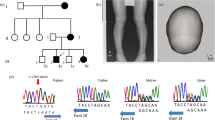
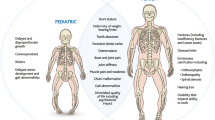
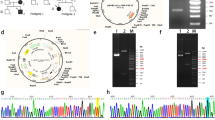
N-ethyl-N-nitrosourea (ENU) mutagenesis is a phenotype-driven approach with potential to assign function to every locus in the mouse genome. In this article, we describe a new mutation, Pug, as a mouse model for X-linked hypophosphatemic rickets (XLH) in human. Mice carrying the Pug mutation exhibit abnormal phenotypes including growth retardation, hypophosphatemia and decreased bone mineral density (BMD). The new mutation was mapped to X-chromosome between 65.4 cM and 66.6 cM, where Phex gene resides. Sequence analysis revealed a unique T-to-C transition mutation resulting in Phe-to-Ser substitution at amino acid 80 of PHEX protein. In vitro studies of Pug mutation demonstrated that PHEXpug was incompletely glycosylated and sequestrated in the endoplasmic reticulum region of cell, whereas wild-type PHEX could be fully glycosylated and transported to the plasma membrane to exert its function as an endopeptidase. Taken together, the Pug mutant directly confirms the role of Phex in phosphate homeostasis and normal skeletal development and may serves as a new disease model of human hypophosphatemic rickets.
Similar content being viewed by others
References
Herron B.J., Lu W., Rao C., Liu S., Peters H., Bronson R.T., Justice M.J., McDonald J.D., Beier D.R. Efficient generation and mapping of recessive developmental mutations using ENU mutagenesis. Nat.Genet. 30:185–189, 2002
Nolan P.M., Peters J., Strivens M., Rogers D., Hagan J., Spurr N., Gray I.C., Vizor L., Brooker D., Whitehill E., Washbourne R., Hough T., Greenaway S., Hewitt M., Liu X., McCormack S., Pickford K., Selley R., Wells C., Tymowska-Lalanne Z., Roby P., Glenister P., Thornton C., Thaung C., Stevenson J.A., Arkell R., Mburu P., Hardisty R., Kiernan A., Erven A., Steel K.P., Voegeling S., Guenet J.L., Nickols C., Sadri R., Nasse M., Isaacs A., Davies K., Browne M., Fisher E.M., Martin J., Rastan S., Brown S.D., Hunter J. A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat. Genet. 25:440–443, 2000
Balling R. ENU mutagenesis: analyzing gene function in mice. Annu. Rev. Genomics Hum. Genet. 2:463–492, 2001
Jan de Beur S.M., Levine M.A. Molecular pathogenesis of hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 87:2467–2473, 2002
Rowe P.S. The wrickkened pathways of FGF23, MEPE and PHEX. Crit. Rev. Oral Biol. Med. 15:264–281, 2004
Rowe P.S., Goulding J., Read A., Mountford R., Hanauer A., Oudet C., Whyte M.P., Meier-Ewert S., Lehrach H., Davies K.E., et al. New markers for linkage analysis of X-linked hypophosphataemic rickets. Hum. Genet. 91:571–575, 1993
HYP-Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 11:130–136, 1995
Rowe P.S., Oudet C.L., Francis F., Sinding C., Pannetier S., Econs M.J., Strom T.M., Meitinger T., Garabedian M., David A., Macher M.A., Questiaux E., Popowska E., Pronicka E., Read A.P., Mokrzycki A., Glorieux F.H., Drezner M.K., Hanauer A., Lehrach H., Goulding J.N., O’Riordan J.L. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP). Hum. Mol. Genet. 6:539–549, 1997
Filisetti D., Ostermann G., von Bredow M., Strom T., Filler G., Ehrich J., Pannetier S., Garnier J.M., Rowe P., Francis F., Julienne A., Hanauer A., Econs M.J., Oudet C. Non-random distribution of mutations in the PHEX gene, and under-detected missense mutations at non-conserved residues. Eur. J. Hum. Genet. 7:615–619, 1999
D’Adamio L., Shipp M.A., Masteller E.L., Reinherz E.L. Organization of the gene encoding common acute lymphoblastic leukemia antigen (neutral endopeptidase 24.11): multiple miniexons and separate 5′ untranslated regions. Proc Natl Acad Sci USA 86:7103–7107, 1989
Xu D., Emoto N., Giaid A., Slaughter C., Kaw S., deWit D., Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell 78:473–485, 1994
Lee S., Zambas E.D., Marsh W.L., Redman C.M. Molecular cloning and primary structure of Kell blood group protein. Proc Natl Acad Sci USA 88:6353–6357, 1991
Francis F., Strom T.M., Hennig S., Boddrich A., Lorenz B., Brandau O., Mohnike K.L., Cagnoli M., Steffens C., Klages S., Borzym K., Pohl T., Oudet C., Econs M.J., Rowe P.S., Reinhardt R., Meitinger T., Lehrach H. Genomic organization of the human PEX gene mutated in X-linked dominant hypophosphatemic rickets. Genome Res. 7:573–585, 1997
Strom T.M., Francis F., Lorenz B., Boddrich A., Econs M.J., Lehrach H., Meitinger T. Pex gene deletions in Gy and Hyp mice provide mouse models for X-linked hypophosphatemia. Hum. Mol. Genet. 6:165–171, 1997
Ruchon A.F., Marcinkiewicz M., Siegfried G., Tenenhouse H.S., DesGroseillers L., Crine P., Boileau G. Pex mRNA is localized in developing mouse osteoblasts and odontoblasts. J. Histochem. Cytochem. 46:459–468, 1998
Beck L., Soumounou Y., Martel J., Krishnamurthy G., Gauthier C., Goodyer C.G., Tenenhouse H.S. Pex/PEX tissue distribution and evidence for a deletion in the 3’ region of the Pex gene in X-linked hypophosphatemic mice. J. Clin. Invest. 99:1200–1209, 1997
Lipman M.L., Panda D., Bennett H.P., Henderson J.E., Shane E., Shen Y., Goltzman D., Karaplis A.C. Cloning of human PEX cDNA. Expression, subcellular localization, and endopeptidase activity. J. Biol. Chem. 273:13729–13737, 1998
Du L., Desbarats M., Viel J., Glorieux F.H., Cawthorn C., Ecarot B. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics 36:22–28, 1996
Guo R., Quarles L.D. Cloning and sequencing of human PEX from a bone cDNA library: evidence for its developmental stage-specific regulation in osteoblasts. J. Bone Miner. Res. 12:1009–1017, 1997
Lajeunesse D., Meyer R.A. Jr., Hamel L. Direct demonstration of a humorally-mediated inhibition of renal phosphate transport in the Hyp mouse. Kidney Int. 50:1531–1538, 1996
Jonsson K.B., Zahradnik R., Larsson T., White K.E., Sugimoto T., Imanishi Y., Yamamoto T., Hampson G., Koshiyama H., Ljunggren O., Oba K., Yang I.M., Miyauchi A., Econs M.J., Lavigne J., Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348:1656–1663, 2003
Strewler G.J. FGF23, hypophosphatemia, and rickets: has phosphatonin been found? Proc. Natl. Acad. Sci. USA 98:5945–5946, 2001
Benet-Pages A., Lorenz-Depiereux B., Zischka H., White K.E., Econs M.J., Strom T.M. FGF23 is processed by proprotein convertases but not by PHEX. Bone 35:455–462, 2004
Liu S., Guo R., Simpson L.G., Xiao Z.S., Burnham C.E., Quarles L.D. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 278:37419–37426, 2003
Eicher E.M., Southard J.L., Scriver C.R., Glorieux F.H. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc. Natl. Acad. Sci. USA 73:4667–4671, 1976
Lyon M.F., Scriver C.R., Baker L.R., Tenenhouse H.S., Kronick J., Mandla S. The Gy mutation: another cause of X-linked hypophosphatemia in mouse. Proc. Natl. Acad. Sci. USA 83:4899–4903, 1986
Carpinelli M.R., Wicks I.P., Sims N.A., O’Donnell K., Hanzinikolas K., Burt R., Foote S.J., Bahlo M., Alexander W.S., Hilton D.J. An ethyl-nitrosourea-induced point mutation in phex causes exon skipping, x-linked hypophosphatemia, and rickets. Am. J. Pathol. 161:1925–1933, 2002
Lorenz-Depiereux B., Guido V.E., Johnson K.R., Zheng Q.Y., Gagnon L.H., Bauschatz J.D., Davisson M.T., Washburn L.L., Donahue L.R., Strom T.M., Eicher E.M. New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm. Genome 15:151–161, 2004
He F., Wang Z., Zhao J., Bao J., Ding J., Ruan H.B. Large-scale screening of disease model through ENU mutagenesis in mice. Chin. Sci. Bull. 48:2665–2671, 2003
Suzuki T., Fujikura K., Higashiyama T., Takata K. DNA staining for fluorescence and laser confocal microscopy. J. Histochem. Cytochem. 45:49–53, 1997
Deng Y., Bennink J.R., Kang H.C., Haugland R.P., Yewdell J.W. Fluorescent conjugates of brefeldin A selectively stain the endoplasmic reticulum and Golgi complex of living cells. J. Histochem. Cytochem. 43:907–915, 1995
Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J. Cell Biol. 85:429–434, 1980
Sabbagh Y., Boileau G., DesGroseillers L., Tenenhouse H.S. Disease-causing missense mutations in the PHEX gene interfere with membrane targeting of the recombinant protein. Hum. Mol. Genet. 10:1539–1546, 2001
Sabbagh Y., Gauthier C., Tenenhouse H.S. The X chromosome deletion in HYP mice extends into the intergenic region but does not include the SAT gene downstream from Phex. Cytogenet. Genome Res. 99:344–349, 2002
Lorenz B., Francis F., Gempel K., Boddrich A., Josten M., Schmahl W., Schmidt J., Lehrach H., Meitinger T., Strom T.M. Spermine deficiency in Gy mice caused by deletion of the spermine synthase gene. Hum. Mol. Genet. 7:541–547, 1998
Miao D., Bai X., Panda D.K., Karaplis A.C., Goltzman D., McKee M.D. Cartilage abnormalities are associated with abnormal Phex expression and with altered matrix protein and MMP-9 localization in Hyp mice. Bone 34:638–647, 2004
Sabbagh Y., Carpenter T.O., Demay M.B. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl. Acad. Sci. USA 102:9637–9642, 2005
Nesbitt T., Fujiwara I., Thomas R., Xiao Z.S., Quarles L.D., Drezner M.K. Coordinated maturational regulation of PHEX and renal phosphate transport inhibitory activity: evidence for the pathophysiological role of PHEX in X-linked hypophosphatemia. J. Bone Miner. Res. 14:2027–2035, 1999
Xiao Z.S., Crenshaw M., Guo R., Nesbitt T., Drezner M.K., Quarles L.D. Intrinsic mineralization defect in Hyp mouse osteoblasts. Am. J. Physiol. 275:E700–E708, 1998
ADHR-Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26:345–348, 2000
Rowe P.S., de Zoysa P.A., Dong R., Wang H.R., White K.E., Econs M.J., Oudet C.L. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics 67:54–68, 2000
Campos M., Couture C., Hirata I.Y., Juliano M.A., Loisel T.P., Crine P., Juliano L., Boileau G., Carmona A.K. Human recombinant endopeptidase PHEX has a strict S1’ specificity for acidic residues and cleaves peptides derived from fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein. Biochem. J. 373:271–279, 2003
Guo R., Rowe P.S., Liu S., Simpson L.G., Xiao Z.S., Quarles L.D. Inhibition of MEPE cleavage by Phex. Biochem. Biophys. Res. Commun. 297:38–45, 2002
Acknowledgements
We thank Jishuai Zhang of the Institute of Biotechnology for technical assistance, as well as Chen Liu of the Gulou Hospital for serum assays. We also thank Drs. Wen Ning and Jiong Chen for their careful review of this manuscript. This work was supported in part by E-Institutes of Shanghai Municipal Education Commission (E03003), Program for Changjiang Scholars and Innovative Research Team in University (IRT0430), and National 973 Projects (2005CB522501 and 2006CB943500) to Xiang Gao.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, X., Qi, X., Ge, X. et al. A novel Phex mutation with defective glycosylation causes hypophosphatemia and rickets in mice. J Biomed Sci 15, 47–59 (2008). https://doi.org/10.1007/s11373-007-9199-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11373-007-9199-6