Summary
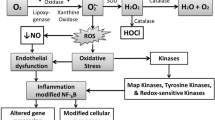
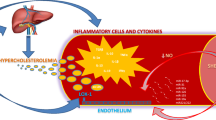
The development of atherosclerotic disease results from the interaction between environment and genetic make up. A key factor in atherogenesis is the oxidative modification of lipids, which is involved in the recruitment of mononuclear leukocytes to the arterial intima – a process regulated by several groups of adhesion molecules and cytokines. Activated leukocytes, as well as endothelial mitochondria, can produce reactive oxygen species (ROS) that are associated with endothelial dysfunction, a cause of reduced nitric oxide (NO) bioactivity and further ROS production. Peroxisome proliferator-activated receptors (PPAR) and liver X receptors (LXR) are nuclear receptors significantly involved in the control of lipid metabolism, inflammation and insulin sensitivity. Also, an emerging role has been suggested for G protein coupled receptors and for the small Ras and Rho GTPases in the regulation of the expression of endothelial NO synthase (eNOS) and of tissue factor, which are involved in thrombus formation and modulation of vascular tone. Further, the interactions among eNOS, cholesterol, oxidated LDL and caveola membranes are probably involved in some molecular changes observed in vascular diseases. Despite the relevance of oxidative processes in atherogenesis, anti-oxidants have failed to significantly improve atherosclerosis (ATS) prevention, while statins have proved to be the most successful drugs.
Similar content being viewed by others
References
Goldstein JL, Brown MS. (1979).The LDL receptor locus and the genetics of familial hypercholesterolemia. Annu Rev Genet. 13:259–89
Jukema JW, van Boven AJ, Groenemeijer B, Zwinderman AH, Reiber JH, Bruschke AV, Henneman JA, Molhoek GP, Bruin T, Jansen H, Gagne E, Hayden MR, Kastelein JJ. (1996). The Asp9 Asn mutation in the lipoprotein lipase gene is associated with increased progression of coronary atherosclerosis. REGRESS Study Group, Interuniversity Cardiology Institute, Utrecht, The Netherlands. Regression Growth Evaluation Statin Study. Circulation 94:193–1918
Mailly F, Tugrul Y, Reymer PW, Bruin T, Seed M, Groenemeyer BF, Asplund-Carlson A, Vallance D, Winder AF, Miller GJ. (1995). A common variant in the gene for lipoprotein lipase (Asp9→Asn). Functional implications and prevalence in normal and hyperlipidemic subjects. Arterioscler Thromb Vasc Biol 15:468–78
Pimstone SN, Gagne SE, Gagne C, Lupien PJ, Gaudet D, Williams RR, Kotze M, Reymer PW, Defesche JC, Kastelein JJ. (1995). Mutations in the gene for lipoprotein lipase. A cause for low HDL cholesterol levels in individuals heterozygous for familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 15:1704–12
Dzau VJ, Gibbons GH, Cooke JP, Omoigui N. (1993). Vascular biology and medicine in the 1990s. scope, concepts, potentials and perspectives. Circulation 87:705–19
van den Ende A, van der Hoek YY, Kastelein JJ, Koschinsky ML, Labeur C, Rosseneu M. (1996). Lipoprotein [a]. Adv Clin Chem 32:73–134
Ribichini F, Steffenino G, Dellavalle A, Matullo G, Colajanni E, Camilla T, Vado A, Benetton G, Uslenghi E, Piazza A. (1998). Plasma activity and insertion/deletion polymorphism of angiotensin I-converting enzyme. a major risk factor and a marker of risk for coronary stent restenosis. Circulation 97:147–54
Fross P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP. (1995). A candidate genetic risk factor for vascular disease. a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–3
Kluijtmans LA, Kastelein JJ, Lindemans J, Boers GH, Heil SG, Bruschke AV, Jukema JW, van den Heuvel LP, Trijbels FJ, Boerma GJ, Verheugt FW, Willelms F, Blom HJ. (1997). Thermolabile methylenetetrahydrofolate reductase in coronary artery disease. Circulation 96:2573–7
Lusis AJ. (2000) Atherosclerosis. Nature 407: 233–241
Doevendans PA, Jukema W, Spiering W, Defesche JC, Kastelein JJP. (2001). Molecular genetics and gene expression in atherosclerosis. International Journal of Cardiology 80:161–172
Puddu P, Cravero E, Puddu GM, Muscari A. (2005). Genes and atherosclerosis: at the origin of the predisposition. Int J Clin Pract 59:462–472
Puddu P, Puddu GM, Muscari A. (2003). Peroxisome proliferator–activated receptors: are they involved in atherosclerosis progression? Int J Cardiol 90:133–140
Li AC, Glass CK. (2004). PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J Lipid Res. 45:2161–73
Marx N, Duez H, Fruchart JC, Staels B.(2004). Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 14(94):1168–78
Neve BP, Fruchart JC, Staels B. (2000). Role of peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol 60:1245–1250
Duez H, Fruchart JC, Staels B. (2001). PPAR in inflammation, atherosclerosis and thrombosis. J Cardiovasc Risk 8:187–194
Elangbam CS, Tyler RD, Lightfoot RM. (2001). Peroxisome proliferator-activated receptors in atherosclerosis and inflammation-an update. Toxicol Pathol 29:224–231
van Raalte DH, Li M, Pritchard PH, Wasan KM. (2004). Peroxisome proliferator-activated receptor (PPAR)-alpha: a pharmacological target with a promising future. Pharm Res. 21:1531–8
Chinetti G, Lestavel S, Fruchart JC, Clavey V, Staels B. (2003). Peroxisome proliferator-activated receptor alpha reduces cholesterol esterification in macrophages. Circ Res. 92:212–7
Israelian-Konaraki Z, Reaven PD. (2005). Peroxisome proliferator-activated receptor-alpha and atherosclerosis: from basic mechanisms to clinical implications. Cardiology. 103:1–9
Collins AR. (2003). Pleiotropic vascular effects of PPARgamma ligands. Drug News Perspect. 16:197–204
Lee DL, Webb RC, Jin L. (2004). Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 44:796–9
Jaye M. (2003). LXR agonists for the treatment of atherosclerosis. Curr Opin Investig Drugs. 4:1053–8
Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. (2002). Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 277:18793–800
Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. (2000). Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14:2819–30
Mak PA, Laffitte BA, Desrumaux C, Joseph SB, Curtiss LK, Mangelsdorf DJ, Tontonoz P, Edwards PA. (2002). Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors alpha and beta. J Biol Chem. 277:31900–8
Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. (2002). Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 99:7604–9
Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. (2002). Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 99:11896–901
Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. (2000). Role of LXRs in control of lipogenesis. Genes Dev. 14:2831–8
Stocker R, Keaney JF, Jr (2004). Role of oxidative modifications in atherosclerosis. Physiol Rev. 84:1381–478
Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. (2004). The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007
Madamanchi NR, Vendrov A, Runge MS. (2005). Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 25:29–38
34. Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW.(2004). The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 31(101):13032–7
Chisolm GM, Steinberg D. (2000). The oxidative modification hypothesis of atherogenesis. an overview. Free Radic Biol Med 28:1815–26
Harrison DG. (1997). Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100:2153–2157
Guzik TJ, West NEJ, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. (2000). Vascular superoxide production by NAD(P)H oxidase. Associaton with endothelial dysfunction and clinical risk factors. Circ Res 86:e85–e90
Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. (1996). Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. J Clin Invest 97:1916–1923
Ross R. (1993). The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 362:801–9
Berliner JA, Heinecke JW. (1996). The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 20:707–27
Freeman BA, White CR, Gutierrez H, Paler-Martinez A, Tarpey MM, Rubbo H. (1995). Oxygen radical-nitric oxide reactions in vascular diseases. Adv Pharmacol. 34:45–69
Alexander RW. (1998). Atherosclerosis as disease of redox-sensitive genes. Trans Am Clin Climatol Assoc. 109:129–45
Ito H, Torii M, Suzuki T. (1995). Decreased superoxide dismutase activity and increased superoxide anion production in cardiac hypertrophy of spontaneously hypertensive rats. Clin Exp Hypertens. 17:803–16
van Jaarsveld H, Kuyl JM, Alberts DW. (1992). Exposure of rats to low concentration of cigarette smoke increases myocardial sensitivity to ischaemia/reperfusion. Basic Res Cardiol. 87:393–9
Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. (1999). Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 96:4820–5
Wallace DC. (1992). Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science 256:628–32
Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. (2002). Mitochondrial integrity and function in atherogenesis. Circulation 106:544–9
Puddu P, Puddu GM, Cravero E, Muscari A. (2005). Mitochondrial dysfunction as an initiating event in atherogenesis - a plausible hypothesis. Cardiology 103:137–141
Ramachandran A, Levonen AL, Brookes PS, Ceaser E, Shiva S, Barone MC, Darley-Usmar V. (2002). Mitochondria, nitric oxide, and cardiovascular dysfunction. Free Radic Biol Med. 33:1465–74
Chance B, Sies H, Boveris A. (1979). Hydroperoxide metabolism in mammalian organs. Physiol Rev. 59:527–605
Papa S, Skulachev VP. (1997). Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem. 174:305–19
Shackelford RE, Kaufmann WK, Paules RS. (2000). Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med 28:1387–404
Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. (2000). Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 86:960–6
Yan LJ, Sohal RS. (1998). Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci U S A. 95:12896–901
MacMillan-Crow LA, Crow JP, Thompson JA. (1998). Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 37:1613–22
Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. (2002). Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 105:849–54
Dart AM, Chin-Dusting JPF. (1999). Lipids and the endothelium. Cardiovasc Res 43:308–322
Lewis TV, Dart AM, Chin-Dusting JPF. (1999). Endothelium-dependent relaxation by acetylcholine is impaired in hypertriglyceridemic humans with normal levels of plasma LDL cholesterol. J Am Coll Cardiol 33:805–12
Parhami F, Fang ZT, Fogelman AM, Andalibi A, Territo MC, Berliner JA. (1993). Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest 92:471–478
Vora DK, Fang ZT, Liva SM, Tyner TR, Parhami F, Watson AD, Drake TA, Territo MC, Berliner JA. (1997). Induction of P-selectin by oxidized lipoproteins. Separate effects on synthesis and surface expression. Circ Res 80:810–818
Smalley DM, Lin JH, Curtis ML, Kobari Y, Stemerman MB, Pritchard KA, Jr.(1996). Native LDL increase endothelial cell adhesiveness by inducing intercellular adhesion molecule-1. Arterioscler Thromb Vasc Biol 16:585–590
Lin JH, Zhu Y, Liao HL, Kobari Y, Groszek L, Stemerman MB. (1996). Induction of vascular cell adhesion molecule-1 by low-density lipoprotein. Atherosclerosis (1996). 127:185–194
Allen S, Khan S, Al-Mohanna F, Batten P, Yacoub M. (1998). Native low-density lipoprotein-induced calcium transient trigger VCAM-1 and E-selectin expression in cultured human vascular endothelial cells. J Clin Invest 101:1064–1075
Rajavashisth TB, Andalibi A, Territo MC, Berliner JA, Navab M, Fogelman AM, Lusis AJ. (1990). Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature 344:254–247
Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. (1994). Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques.Coexpression with intercellular adhesion molecule-1. Am J Pathol 144:952–961
Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. (1996). Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA 93:9114–9119
Sugiyama S, Kugiyama K, Ohgushi M, Fujimoto K, Yasue H. (1994). Lysophosphatidylcholine in oxidized low-density lipoprotein increases endothelial susceptibility to polymorphonuclear leukocyte-induced endothelial dysfunction in porcine coronary arteries. Role of protein kinase C. Circ Res 74:565–575
Maier JA, Barenghi L, Bradamante S, Pagani F. (1994). Modulators of oxidized LDL-induced hyperadhesiveness in human endothelial cells. Biochem Biophys Res Commun 204:673–677
Myers DE, Huang WN, Larkins RG. (1996). Lipoprotein-induced prostacyclin production in endothelial cells and effects of lipoprotein modification. Am J Physiol 271:C1504–1511
Haug C, Schmid-Kotsas A, Zorn U, Schuett S, Gross HJ, Gruenert A, Bachem MG. (2001). Endothelin-1 synthesis and endothelin b receptor expression in human coronary artery smooth muscle cells and monocyte-derived macrophages is up-regulated by low density lipoproteins. J Mol Cell Cardiol 33:1701–12
He Y, Kwan WC, Steinbrecher UP. (1996). Effects of oxidized low density lipoprotein on endothelin secretion by cultured endothelial cells and pacrophages. Atherosclerosis 119:107–118
Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA. (1991). Role of basic fibroblast growth factor in vascular lesion formation. Circ Res 68:106–113
Stiko-Rahm A, Hultgardh-Nilsson A, Regnstrom J, Hamsten A, Nilsson J. (1992). Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. Arterioscler Thromb 12:1099–1109
Kohno M, Yokokawa K, Yasunari K, Minami M, Kano H, Hanehira T, Yoshikawa J. (1998). Induction by lysophosphatidylcholine, a major phospholipid component of atherogenic lipoproteins, of human coronary artery smooth muscle cell migration. Circulation 98:353–359
Kim JG, Taylor WR, Parthasarathy S. (1999). Demonstration of the presence of lipid peroxide-modified proteins in human atherosclerotic lesions using a novel lipid peroxidemodified anti-peptide antibody. Atherosclerosis 143:335–340
Mertens A, Holvoet P. (2001). Oxidized LDL and HDL. antagonists in atherothrombosis. FASEB J 15:2073–2084
Thorin E, Hamilton CA, Dominiczak MH, Reid JL. (1994). Chronic exposure of cultured bovine endothelial cells to oxidized LDL abolishes prostacyclin release. Arterioscler Thromb 14:453–459
Li LX, Chen JX, Liao DF, Yu L. (1998). Probucol inhibits oxidized-low density lipoprotein-induced adhesion of monocytes to endothelial cells by reducing P-selectin synthesis in vitro. Endothelium 6:1–8
Frei B, Gaziano JM. (1993). Content of antioxidants, preformed lipid hydroperoxides and cholesterol as predictors of the susceptibility of human LDL to metal ion-dependent and-independent oxidation. J Lipid Res 34:2135–2145
Penn MS, Vui MZ, Winokur AL, Bethea J, Hamilton TA, DiCorleto PE, Chisolm GM. (2000). Smooth muscle cell surface tissue factor pathway activation by oxidized low-density lipoprotein requires cellular lipid peroxidation. Blood 96:30556–3063
Ishii H, Kizaki K, Horie S, Kazama M. (1996). Oxidized low density lipoprotein reduces thrombomodulin transcription in cultured human endothelial cells through degradation of the lipoprotein in lysosomes. J Biol Chem 271:8458–8465
Kockx MM. (1998). Apoptosis in the atherosclerotic plaque. quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol 18:1519–1522
Heermeier K, Leicht W, Palmetshofer A, Ullrich M, Wanner C, Galle J. (2001). Oxidized LDL suppresses NF-kappaB and overcomes protection from apoptosis in activated endothelial cells. J Am Soc Nephrol 12:456–463
Metzler B, Hu Y, Dietrich H, Xu Q. (2000). Increased expression and activation of stress-activated protein kinases/c-Jun NH (2)-terminal protein kinases in atherosclerotic lesions coincide with p53. Am J Pathol 156:1875–1886
Carlos TM, Harlan JM. (1994). Leukocyte-endothelial adhesion molecules. Blood 84:2068–101
Frenette P, Wagner D. (1996). Adhesion molecules. New Engl J Med 334:1526–29
Vora DK, Fang ZT, Liva SM, Tyner TR, Parhami F, Watson AD, Drake TA, Territo MC, Berliner JA. (1997). Induction of P-selectin by oxidized lipoproteins. Separate effects on synthesis and surface expression. Circ Res 80:810–18
Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. (1998). The combined role of P- and E-selectins in atherosclerosis. J Clin Invest 102:145–52
Frenette PS, Wagner DD. (1997). Insights into selectin function from knockout mice. Thromb Haemost 78:60–64
Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD. (1997). Absence of P-selectin delays fatty streak formation in mice. J Clin Invest 99:1037–43
Cybulsky MI, Gimbrone MA , Jr.(1991). Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251:788–91
Li H, Cybulsky MI, Gimbrone MA Jr, Libby P. (1991). An atherogenic diet rapidly induces VCAM-1, a cytokine regulatable mononuclear leukocyte adhesion molecule, in rabbit endothelium. Arterioscler Thromb (1993). 13:197–204
Iiyama K, Hajra L, Iiyama M, Li H, Di Chiara M, Medoff BD, Cybulsky MI. (1999). Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 85:199–207
Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. (1995). Targeted disruption of the murine VCAM1 gene. essential role of VCAM-1 in choriooallantoic fusion and placentation. Genes Dev 9:1–14
Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. (1998). Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low-density lipoprotein-deficient mice. Mol Cell 2:275–81
Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. (2004). Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 378:539–47
Eriksson EE. (2004). Mechanisms of leukocyte recruitment to atherosclerotic lesions: future prospects. Curr Opin Lipidol. 15:553–558
Witztum JL, Berliner JA. (2004). Oxodized phospholipids and isoprostanes in atherosclerosis. Curr Opin Lipidol (1998). 9:441–48
Gimbrone MA Jt, Nagel T, Topper JN. (1997). Biomechanical activation. an emerging paradigm in endothelial adhesion biology. J Clin Invest 100:S61–65
Collins T. (1993). Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest 68:499–508
Boring L, Gosling J, Cleary M, Charo IF. (1998). Decreased lesion formation in CCR2-/-mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394:894–97
Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. (1997). Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272:13 597–607
Wheeler AP, Ridley AJ. (2004). Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res 301:43–9
Van Aelst L, D’Souza-Schorey C. (1997). Rho GTPases and signaling networks. Genes Dev. 11:2295–322
Scita G, Tenca P, Frittoli E, Tocchetti A, Innocenti M, Giardina G, Di Fiore PP. (2000). Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 19:2393–8
Danesh F.R., Sadeghi M.M., Amro N., Philips C., Zeng L., Lin S., Sahai A. and Kanwar Y.S., 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/ p21 signaling pathway: Implications for diabetic nephropathy. Proc. Natl. Acad. Sci. USA 99: 8301–8305, 2002 (Epub 2002 Jun 04)
Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. (2002). Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. 105:1756–9
Endres M, Laufs U. (2004). Effects of statins on endothelium and signaling mechanisms. Stroke 35:2708–11
Ruiz-Velasco N, Dominguez A (2004). Vega.Statins upregulate CD36 expression in human monocytes, an effect strengthened when combined with PPAR-gamma ligands Putative contribution of Rho GTPases in statin-induced CD36 expression. Biochem Pharmacol. 67:303–313
Bhattacharya M, Babwah AV, Ferguson SS. (2004). Small GTP-binding protein-coupled receptors. Biochem Soc Trans 2004;32:1040–4
Laufs U, Liao JK. (1998). Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 273:24266–71
Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E, Allen PG, Huang PL, Bohm M, Schoen FJ, Moskowitz MA, Liao JK. (2000). Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 106:15–24
Goldstein JL, Brown MS. (1990). Regulation of the mevalonate pathway. Nature. 343:425–30
Park HJ, Kong D, Iruela-Arispe L, Begley U, Tang D, Galper JB. (2002). 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res. 91:143–50
Wassmann S, Laufs U, Baumer AT, Muller K, Konkol C, Sauer H, Bohm M, Nickenig G. (2001). Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 59:646–54
Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. (2000). Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 20:61–9
Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. (2000). A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 87:26–32
Ishibashi T, Nagata K, Ohkawara H, Sakamoto T, Yokoyama K, Shindo J, Sugimoto K, Sakurada S, Takuwa Y, Teramoto T, Maruyama Y. (2002). Inhibition of Rho/Rho-kinase signaling downregulates plasminogen activator inhibitor-1 synthesis in cultured human monocytes. Biochim Biophys Acta. 1590:123–30
Marrero MB, Venema VJ, Ju H, He H, Liang H, Caldwell RB, Venema RC. (1999). Endothelial nitric oxide synthase interactions with G-protein–coupled receptors. Biochem J 343:335–40
Tsutsui M, Shimokawa H, Tanaka S, Kuwaoka I, Hase K, Nogami N, Nakanishi K, Okamatsu S. (1994). Endothelial Gi protein in human coronary arteries. Eur Heart J 15:1261–6
Yang CM, Chien CS, Hsiao LD, Pan SL, Wang CC, Chiu CT, Lin CC. (2001). Mitogenic effect of oxidized low-density lipoprotein on vascular smooth muscle cells mediated by activation of Ras/Raf/MEK/MAPK pathway. Br J Pharmacol 132:1531–41
Yang CM, Chiu CT, Wang CC, Chien CS, Hsiao LD, Lin CC, Tu MT, Pan SL. (2000). Activation of mitogen-activated protein kinase by oxidized low-density lipoprotein in canine cultured vascular smooth muscle cells. Cell Signal 12:205–14
Zhao D, Letterman J, Schreiber BM. (2001). b-migrating very low density lipoprotein (bVLDL) activates smooth muscle cell mitogen-activated protein (MAP) kinase via G protein-coupled receptor-mediated transactivation of the epidermal growth factor (EGF) receptor. J Biol Chem 276:30579–30588
Uittenbogaard A, Smart EJ. (2000). Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J Biol Chem 275:255595–9
Everson WV, Smart EJ. (2001). Influence of caveolin, cholesterol and lipoproteins on nitric oxide synthase. Implications for vascular disease. Trends Cardiovasc Med 11:246–250
Uittenbogaard A, Ying Y, Smart EJ. (1998). Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem 273:6525–32
Stein O, Stein Y. (1999). Atheroprotective mechanisms of HDL. Atherosclerosis 144:285–303
Von Eckardstein A, Assmann G. (2000). Prevention of coronary heart disease by raising of high density lipoprotein cholesterol?. Curr Opin Lipidol 11:627–637
Toikka JO, Ahotupa M, Viikari JS, Niinikoski H, Taskinen M, Irjala K, Hartiala JJ, Raitakari OT. (1999). Constantly low HDL-cholesterol concentration relates to endothelial dysfunction and increased in vivo LDL-oxidation in healthy young men. Atherosclerosis 147:133–138
Krause BR, Auerbach BJ. (2001). Reverse cholesterol transport and future pharmacological approaches to the treatment of atherosclerosis. Curr Opin Investig Drugs 2:375–81
von Eckardstein A, Nofer JR, Assmann G. (2001). High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol 21:13–27
Fazio S. (1997). Increased atherogenesis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci USA 94:4647–4652
Ohgami N, Nagai R, Miyazaki A, Ikemoto M, Arai H, Horiuchi S, Nakayama H. (2001). Scavenger receptor class B type-I-mediated reverse cholesterol transport is inhibited by advanced glycation end products. J Biol Chem 276:13348–55
Wade DP, Owen JS. (2001). Regulation of the cholesterol efflux gene, ABCA1. Lancet 357:161–163
Wang N, Silver DL, Thiele C, Tall AR. (2001). ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 276:23742–23747
Frank PG, Galbiati F, Volonte D, Razani B, Cohen DE, Marcel YL, Lisanti MP. (2001). Influence of caveolin-1 on cellular cholesterol efflux mediated by high-density lipoproteins. Am J Physiol Cell Physiol 280:C1204–14
Oliver WR Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. (2001). A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA 98:5306–11
Fazio S, Linton MF. (2001). The inflamed plaque.cytokine production and cellular cholesterol balance in the vessel wall. Am J Cardiol 88:12E–15E
Yamashita S, Hirano K, Sakai N, Matsuzawa Y. (2000). Molecular biology and pathophysiological aspects of plasma cholesteryl ester transfer protein. Biochim Biophys Acta 1529:257–75
Krieger M. (1999). Charting the fate of the good cholesterol. identification and characterization of the high density lipoprotein receptor SR-BI. Annu Rev Biochem 68:523–558
Trigatti B, Rigotti A, Krieger M. (2000). The role of high-density lipoprotein receptor SR-BI in cholesterol metabolism. Curr Opin Lipidol 11:123–132
Curtiss LK, Boisvert WA. (2000). Apolipoprotein E and atherosclerosis. Curr Opin Lipidol 11:243–251
Tall AR, Jiang XC, Luo Y, Silver D. (2000). George Lyman Duff Memorial Lecture. lipid transfer proteins, HDL metabolism and atherogenesis. Arterioscler Thromb Vasc Biol 20:1185–1188
Cohen J, Vega GL, Grundy SM. (1999). Hepatic lipase. new insights from genetic and metabolic studies. Curr Opin Lipidol 10:259–268
Thuren T. (2000). Hepatic lipase and HDL metabolism. Curr Opin Lipidol 11:277–284
Rader D, Jaye M. (2000). Endothelial lipase. a new member of the triglyceride lipase gene family. Curr Opin Lipidol 11:141–148
VonEckardstein A, Huang Y, Assmann G. (1994). Physiological role and clinical relevance of high-density lipoprotein subclasses. Curr Opin Lipidol 5:404–416
Barrans A, Jaspard B, Barbaras R, Chap H, Perret B, Collet X. (1996). Pre-b HDL. structure and metabolism. Biochim Biophys Acta 1300:73–85
Fielding C, Fielding PE. (1995). Molecular physiology of reverse cholesterol transport. J Lipid Res 36:211–228
Moestrup SK, Kozyraki R. (2000). Cubilin, a high density lipoprotein receptor. Curr Opin Lipidol 11:133–140
Kozyraki R, Fyfe J, Kristiansen M, Gerdes C, Jacobsen C, Cui SY, Christensen EI, Aminoff M, de la Chapelle A, Krahe R et al.(1999) The intrinsic factor-vitamin B-12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat Med 5:656–661
Hammad SM, Steffansson S, Twal WO, Drake CJ, Fleming P, Remaley A, Brewer HB Jr, Argraves WS. (1999). Cubilin, the endocytic receptor for intrinsic factor-vitamin B (12) complex, mediates high-density lipoprotein holoparticle endocytosis. Proc Natl Acad Sci USA . 96:10158–10163
Dhaliwal BS, Steinbrecher UP. (2000). Cholesterol delivered to macrophages by oxidized low density lipoprotein is sequestered in lysosomes and fails to efflux normally. J Lipid Res 41:1658–1665
Aviram M, Rosenblat M. (2004). Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 37:1304–16
Becker AE, de Boer OJ, van der Wal AC. (2001). The role of inflammation and infection in coronary artery disease. Annu Rev Med 52:289–97
Van der Wal AC, Piek JJ, de Boer OJ, Koch KT, Teeling P, van der Loos CM, Becker AE. (1998). Recent activation of the plaque immune response in coronary lesions underlying acute coronary syndromes. Heart 80:14–18
Libby P. (2000). Changing concepts of atherogenesis. J Int Med 247:349–358
Libby P. (1995). Molecular bases of the acute coronary syndromes. Circulation 91:2844–2850
Herman MP, Sukhova GK, Kisiel W, Foster D, Kehry MR, Libby P, Schonbeck U. (2001). Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest 107:1117–26
Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. (1997). Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD-40. induction of collagenase, stromelysin and tissue factor. Circulation 96:396–399
Schonbeck U, Mach F, Sukhova GK, Herman M, Graber P, Kehry MR, Libby P. (2000). CD40 ligation induces tissue factor expression in human vascular smooth muscle cells. Am J Pathol 156:7–14
Marx N, Mackman N, Schonbeck U, Yilmaz N, Hombach VV, Libby P, Plutzky J. (2001). PPARalpha activators inhibit tissue factor expression and activity in human monocytes. Circulation 103:213–219
Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. (2000). Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342:154–60
Heart Protection Study Collaborative Group.(2002). MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360:23–33
Ricciarelli R, Zingg JM, Azzi A. (2001). Vitamin E. protective role of a Janus molecule. FASEB J (2001). 15:2314–2325
Heinecke JW. (2001). Is the emperor wearing clothes? Clinical trials of vitamin E and the LDL oxidation hypothesis. Arterioscler Thromb Vasc Biol 21:1261–1264
Keaney JF, Jr.(2000). Atherosclerosis. from lesion formation to plaque activation and endothelial dysfunction. Mol Aspects Med 21:99–166
Libby P. (2001). Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 104:365–372
Jessup W, Kritharides L, Stocker R. (2004). Lipid oxidation in atherogenesis: an overview. Biochem Soc Trans. 32:134–8
Stocker R, Keaney JF, Jr.(2004). Role of oxidative modifications in atherosclerosis. Physiol Rev. 84:1381–478
Vaughan CJ, Gotto AM, Basson CT. (2000). The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol . 35:1–10
Puddu P, Puddu GM, Muscari A. (2001). Current thinking in statin therapy. Acta Cardiol 56:225–231
Puddu P, Puddu GM, Muscari A. (2001). HMG-CoA reductase inhibitors. is the endothelium the main target? Cardiology 95:9–13
Ambrosi P, Villani P, Habib G, Bouvenot G. (2003). The statins: new properties. Therapie. 58:15–21
Parissis J, Korovesis S, Kalivas P, Giagitzoglou E, Katritsis D. (2000). Short-term atorvastatin therapy reduces monocyte-related inflammatory markers in the serum of hypercholesterolaemic patients. Eur Heart J 21:154
Chase AJ, Newby AC. (2000). Cerivastatin inhibits metalloproteinase production by human vascular smooth muscle cells in response to inflammatory stimuli, a possible mechanism for plaque stabillisation. Eur Heart J 21:152
Sinkiewicz W, Hoffman A, Blazejewski J, Bujak R, Budzynski J, Zbikowska M, Palgan K. (2000). The serum immunoglobulin IgE, interleukin-4 and soluble form receptor CD23 dynamic changes in patients with hypercholesterolaemia II B treated with pravastatin. Eur Heart J 21:155
Paul A, Calleja L, Camps J, Osada J, Vilella E, Ferré N, Mayayo E, Joven J. (2000). The continuous administration of aspirin attenuates atherosclerosis in apolipoprotein E-deficient mice. Life Sci 68:457–465
Fuster V, Dyken ML, Vokonas PS, Hennekens C. (1993). Aspirin as a therapeutic agent in cardiovascular disease. Circulation 87:659–75
Jukema JW. (1999). Matching treatment to the genetic basis of (lipid) disorder in patients with coronary artery disease. Heart 82:126–127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puddu, G., Cravero, E., Arnone, G. et al. Molecular aspects of atherogenesis: new insights and unsolved questions. J Biomed Sci 12, 839–853 (2005). https://doi.org/10.1007/s11373-005-9024-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11373-005-9024-z